An experimental model for studying the biomechanics of embryonic tendon: Evidence that the development of mechanical properties depends on the actinomyosin machinery
- PMID: 20736063
- PMCID: PMC3611596
- DOI: 10.1016/j.matbio.2010.08.009
An experimental model for studying the biomechanics of embryonic tendon: Evidence that the development of mechanical properties depends on the actinomyosin machinery
Abstract
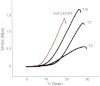
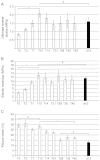
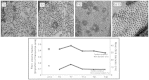
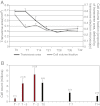
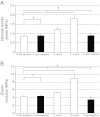
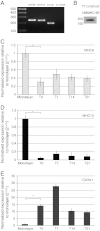
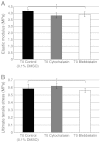
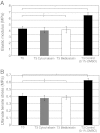
Tendons attach muscles to bone and thereby transmit tensile forces during joint movement. However, a detailed understanding of the mechanisms that establish the mechanical properties of tendon has remained elusive because of the practical difficulties of studying tissue mechanics in vivo. Here we have performed a study of tendon-like constructs made by culturing embryonic tendon cells in fixed-length fibrin gels. The constructs display mechanical properties (toe-linear-fail stress-strain curve, stiffness, ultimate tensile strength, and failure strain) as well as collagen fibril volume fraction and extracellular matrix (ECM)/cell ratio that are statistically similar to those of embryonic chick metatarsal tendons. The development of mechanical properties during time in culture was abolished when the constructs were treated separately with Triton X-100 (to solubilise membranes), cytochalasin (to disassemble the actin cytoskeleton) and blebbistatin (a small molecule inhibitor of non-muscle myosin II). Importantly, these treatments had no effect on the mechanical properties of the constructs that existed prior to treatment. Live-cell imaging and (14)C-proline metabolic labeling showed that blebbistatin inhibited the contraction of the constructs without affecting cell viability, procollagen synthesis, or conversion of procollagen to collagen. In conclusion, the mechanical properties per se of the tendon constructs are attributable to the ECM generated by the cells but the improvement of mechanical properties during time in culture was dependent on non-muscle myosin II-derived forces.
Copyright © 2010 International Society of Matrix Biology. Published by Elsevier B.V. All rights reserved.
Figures



Similar articles
-
The different roles of myosin IIA and myosin IIB in contraction of 3D collagen matrices by human fibroblasts.Exp Cell Res. 2014 Aug 15;326(2):295-306. doi: 10.1016/j.yexcr.2014.04.013. Epub 2014 Apr 25. Exp Cell Res. 2014. PMID: 24768700 Free PMC article.
-
Actin cytoskeleton contributes to the elastic modulus of embryonic tendon during early development.J Orthop Res. 2015 Jun;33(6):874-81. doi: 10.1002/jor.22880. J Orthop Res. 2015. PMID: 25721681 Free PMC article.
-
Blebbistatin, a novel inhibitor of myosin II ATPase activity, increases aqueous humor outflow facility in perfused enucleated porcine eyes.Invest Ophthalmol Vis Sci. 2005 Nov;46(11):4130-8. doi: 10.1167/iovs.05-0164. Invest Ophthalmol Vis Sci. 2005. PMID: 16249490
-
Mammalian nonmuscle myosin II comes in three flavors.Biochem Biophys Res Commun. 2018 Nov 25;506(2):394-402. doi: 10.1016/j.bbrc.2018.03.103. Epub 2018 Mar 17. Biochem Biophys Res Commun. 2018. PMID: 29550471 Free PMC article. Review.
-
Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading.Physiol Rev. 2004 Apr;84(2):649-98. doi: 10.1152/physrev.00031.2003. Physiol Rev. 2004. PMID: 15044685 Review.
Cited by
-
Synchronized mechanical oscillations at the cell-matrix interface in the formation of tensile tissue.Proc Natl Acad Sci U S A. 2018 Oct 2;115(40):E9288-E9297. doi: 10.1073/pnas.1801759115. Epub 2018 Sep 20. Proc Natl Acad Sci U S A. 2018. PMID: 30237286 Free PMC article.
-
A review of methods to measure tendon dimensions.J Orthop Surg Res. 2019 Jan 14;14(1):18. doi: 10.1186/s13018-018-1056-y. J Orthop Surg Res. 2019. PMID: 30636623 Free PMC article. Review.
-
Arhgap28 is a RhoGAP that inactivates RhoA and downregulates stress fibers.PLoS One. 2014 Sep 11;9(9):e107036. doi: 10.1371/journal.pone.0107036. eCollection 2014. PLoS One. 2014. PMID: 25211221 Free PMC article.
-
Mechanical factors in embryonic tendon development: potential cues for stem cell tenogenesis.Curr Opin Biotechnol. 2013 Oct;24(5):834-40. doi: 10.1016/j.copbio.2013.07.003. Epub 2013 Aug 2. Curr Opin Biotechnol. 2013. PMID: 23916867 Free PMC article. Review.
-
Human adipose stromal cells differentiate towards a tendon phenotype with adapted visco-elastic properties in a 3D-culture system.Biol Open. 2025 May 15;14(5):bio061911. doi: 10.1242/bio.061911. Epub 2025 May 12. Biol Open. 2025. PMID: 40271554 Free PMC article.
References
-
- Abe M., Ho C.H., Kamm K.E., Grinnell F. Different molecular motors mediate platelet-derived growth factor and lysophosphatidic acid-stimulated floating collagen matrix contraction. J. Biol. Chem. 2003;278:47707–47712. - PubMed
-
- Allingham J.S., Smith R., Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat. Struct. Mol. Biol. 2005;12:378–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

