Selective inhibition of human 3β-hydroxysteroid dehydrogenase type 1 as a potential treatment for breast cancer
- PMID: 20736065
- PMCID: PMC2999670
- DOI: 10.1016/j.jsbmb.2010.08.003
Selective inhibition of human 3β-hydroxysteroid dehydrogenase type 1 as a potential treatment for breast cancer
Abstract
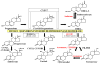
Human 3β-hydroxysteroid dehydrogenase/isomerase type 1 (3β-HSD1) is a critical enzyme in the conversion of DHEA to estradiol in breast tumors and may be a target enzyme for inhibition in the treatment of breast cancer in postmenopausal women. Human 3β-HSD2 participates in the production of cortisol and aldosterone in the human adrenal gland in this population. In our recombinant human breast tumor MCF-7 Tet-off cells that express either 3β-HSD1 or 3β-HSD2, trilostane and epostane inhibit the DHEA-induced proliferation of MCF-7 3β-HSD1 cells with 12-16-fold lower IC(50) values compared to the MCF-7 3β-HSD2 cells. Trilostane and epostane also competitively inhibit purified human 3β-HSD1 with 12-16-fold lower K(i) values compared to the noncompetitive K(i) values measured for human 3β-HSD2. Using our structural model of 3β-HSD1, trilostane was docked in the active site of 3β-HSD1, and Arg195 in 3β-HSD1 or Pro195 in 3β-HSD2 was identified as a potentially critical residue. The R195P-1 mutant of 3β-HSD1 and the P195R-2 mutant of 3β-HSD2 were created, expressed and purified. Kinetic analyses of enzyme inhibition suggest that the high-affinity, competitive inhibition of 3β-HSD1 by trilostane may be related to the presence of Arg195 in 3β-HSD1 versus Pro195 in 3β-HSD2. In addition, His156 in 3β-HSD1 may play a role in the higher affinity of 3β-HSD1 for substrates and inhibitors compared to 3β-HSD2 containing Try156. Structural modeling of the 3β-HSD1 dimer identified a possible interaction between His156 on one subunit and Gln105 on the other. Kinetic analyses of the H156Y-1, Q105M-1 and Q105M-2 support subunit interactions that contribute to the higher affinity of 3β-HSD1 for the inhibitor, epostane, compared to 3β-HSD2. Article from the Special issue on Targeted Inhibitors.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

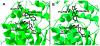

References
-
- Rheaume E, Lachance Y, Zhao HF, Breton N, Dumont M, de Launoit Y, Trudel C, Luu-The V, Simard J, Labrie F. Structure and expression of a new complementary DNA encoding the almost exclusive 3 beta-hydroxysteroid dehydrogenase/delta 5- delta 4-isomerase in human adrenals and gonads. Mol Endocrinol. 1991;5(8):1147–1157. - PubMed
-
- Persson B, Kallberg Y, Bray JE, Bruford E, Dellaporta SL, Favia AD, Duarte RG, Jörnvall H, Kavanagh KL, Kedishvili N, Kisiela M, Maser E, Mindnich R, Orchard S, Penning TM, Thornton JM, Adamski J, Oppermann U. The SDR (short-chain dehydrogenase/reductase and related enzymes) nomenclature initiative. Chem Biol Interact. 2009;178(1–3):94–98. - PMC - PubMed
-
- Thomas JL, Myers RP, Strickler RC. Human placental 3β-hydroxy-5-ene-steroid dehydrogenase and steroid 5–4-ene-isomerase: purification from mitochondria and kinetic profiles, biophysical characterization of the purified mitochondrial and microsomal enzymes. J Steroid Biochem. 1989;33(2):209–217. - PubMed
-
- Kacsoh B. Endocrine Physiology. McGraw-Hill Companies, Inc; New York: 2000. pp. 566–567.
-
- Penning TM, Burczynski ME, Jez JM, Hung CF, Lin HK, Ma H, Moore M, Palackal N, Ratnam K. Human 3alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351(1):67–77. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

