Transamination is required for {alpha}-ketoisocaproate but not leucine to stimulate insulin secretion
- PMID: 20736162
- PMCID: PMC2962470
- DOI: 10.1074/jbc.M110.136846
Transamination is required for {alpha}-ketoisocaproate but not leucine to stimulate insulin secretion
Abstract
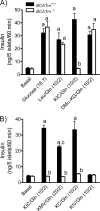
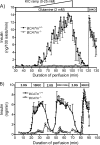
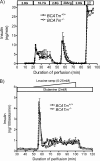
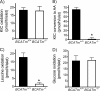
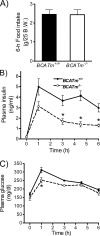
It remains unclear how α-ketoisocaproate (KIC) and leucine are metabolized to stimulate insulin secretion. Mitochondrial BCATm (branched-chain aminotransferase) catalyzes reversible transamination of leucine and α-ketoglutarate to KIC and glutamate, the first step of leucine catabolism. We investigated the biochemical mechanisms of KIC and leucine-stimulated insulin secretion (KICSIS and LSIS, respectively) using BCATm(-/-) mice. In static incubation, BCATm disruption abolished insulin secretion by KIC, D,L-α-keto-β-methylvalerate, and α-ketocaproate without altering stimulation by glucose, leucine, or α-ketoglutarate. Similarly, during pancreas perfusions in BCATm(-/-) mice, glucose and arginine stimulated insulin release, whereas KICSIS was largely abolished. During islet perifusions, KIC and 2 mM glutamine caused robust dose-dependent insulin secretion in BCATm(+/+) not BCATm(-/-) islets, whereas LSIS was unaffected. Consistently, in contrast to BCATm(+/+) islets, the increases of the ATP concentration and NADPH/NADP(+) ratio in response to KIC were largely blunted in BCATm(-/-) islets. Compared with nontreated islets, the combination of KIC/glutamine (10/2 mM) did not influence α-ketoglutarate concentrations but caused 120 and 33% increases in malate in BCATm(+/+) and BCATm(-/-) islets, respectively. Although leucine oxidation and KIC transamination were blocked in BCATm(-/-) islets, KIC oxidation was unaltered. These data indicate that KICSIS requires transamination of KIC and glutamate to leucine and α-ketoglutarate, respectively. LSIS does not require leucine catabolism and may be through leucine activation of glutamate dehydrogenase. Thus, KICSIS and LSIS occur by enhancing the metabolism of glutamine/glutamate to α-ketoglutarate, which, in turn, is metabolized to produce the intracellular signals such as ATP and NADPH for insulin secretion.
Figures
Similar articles
-
Alpha-Ketoisocaproate-induced hypersecretion of insulin by islets from diabetes-susceptible mice.Am J Physiol Endocrinol Metab. 2005 Aug;289(2):E218-24. doi: 10.1152/ajpendo.00573.2004. Epub 2005 Mar 1. Am J Physiol Endocrinol Metab. 2005. PMID: 15741243
-
Distinguishing features of leucine and alpha-ketoisocaproate sensing in pancreatic beta-cells.Endocrinology. 2003 May;144(5):1949-57. doi: 10.1210/en.2002-0072. Endocrinology. 2003. PMID: 12697702
-
Identification of the mitochondrial branched chain aminotransferase as a branched chain alpha-keto acid transport protein.J Biol Chem. 1993 Feb 15;268(5):3084-91. J Biol Chem. 1993. PMID: 8428987
-
An overview of branched-chain amino acid aminotransferases: functional differences between mitochondrial and cytosolic isozymes in yeast and human.Appl Microbiol Biotechnol. 2021 Nov;105(21-22):8059-8072. doi: 10.1007/s00253-021-11612-4. Epub 2021 Oct 8. Appl Microbiol Biotechnol. 2021. PMID: 34622336 Review.
-
Brain amino acid requirements and toxicity: the example of leucine.J Nutr. 2005 Jun;135(6 Suppl):1531S-8S. doi: 10.1093/jn/135.6.1531S. J Nutr. 2005. PMID: 15930465 Review.
Cited by
-
One-step biosynthesis of α-ketoisocaproate from L-leucine by an Escherichia coli whole-cell biocatalyst expressing an L-amino acid deaminase from Proteus vulgaris.Sci Rep. 2015 Jul 28;5:12614. doi: 10.1038/srep12614. Sci Rep. 2015. PMID: 26217895 Free PMC article.
-
PWD/PhJ mice have a genetically determined increase in nutrient-stimulated insulin secretion.Mamm Genome. 2015 Apr;26(3-4):131-41. doi: 10.1007/s00335-015-9554-2. Epub 2015 Jan 21. Mamm Genome. 2015. PMID: 25605412
-
Engineering of L-amino acid deaminases for the production of α-keto acids from L-amino acids.Bioengineered. 2019 Dec;10(1):43-51. doi: 10.1080/21655979.2019.1595990. Bioengineered. 2019. PMID: 30876377 Free PMC article. Review.
-
Cytosolic branched chain aminotransferase (BCATc) regulates mTORC1 signaling and glycolytic metabolism in CD4+ T cells.J Biol Chem. 2014 Jul 4;289(27):18793-804. doi: 10.1074/jbc.M114.554113. Epub 2014 May 20. J Biol Chem. 2014. PMID: 24847056 Free PMC article.
-
Interaction of ingested leucine with glycine on insulin and glucose concentrations.J Amino Acids. 2014;2014:521941. doi: 10.1155/2014/521941. Epub 2014 Jul 10. J Amino Acids. 2014. PMID: 25120925 Free PMC article.
References
-
- Henquin J. C., Dufrane D., Nenquin M. (2006) Diabetes 55, 3470–3477 - PubMed
-
- Hutton J. C., Sener A., Malaisse W. J. (1980) J. Biol. Chem. 255, 7340–7346 - PubMed
-
- Lenzen S., Formanek H., Panten U. (1982) J. Biol. Chem. 257, 6631–6633 - PubMed
-
- Malaisse W. J. (1986) Adv. Enzyme Regul. 25, 203–217 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

