Transamination is required for {alpha}-ketoisocaproate but not leucine to stimulate insulin secretion
- PMID: 20736162
- PMCID: PMC2962470
- DOI: 10.1074/jbc.M110.136846
Transamination is required for {alpha}-ketoisocaproate but not leucine to stimulate insulin secretion
Abstract
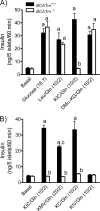
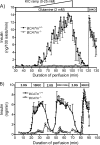
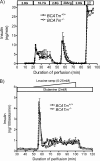
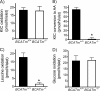
It remains unclear how α-ketoisocaproate (KIC) and leucine are metabolized to stimulate insulin secretion. Mitochondrial BCATm (branched-chain aminotransferase) catalyzes reversible transamination of leucine and α-ketoglutarate to KIC and glutamate, the first step of leucine catabolism. We investigated the biochemical mechanisms of KIC and leucine-stimulated insulin secretion (KICSIS and LSIS, respectively) using BCATm(-/-) mice. In static incubation, BCATm disruption abolished insulin secretion by KIC, D,L-α-keto-β-methylvalerate, and α-ketocaproate without altering stimulation by glucose, leucine, or α-ketoglutarate. Similarly, during pancreas perfusions in BCATm(-/-) mice, glucose and arginine stimulated insulin release, whereas KICSIS was largely abolished. During islet perifusions, KIC and 2 mM glutamine caused robust dose-dependent insulin secretion in BCATm(+/+) not BCATm(-/-) islets, whereas LSIS was unaffected. Consistently, in contrast to BCATm(+/+) islets, the increases of the ATP concentration and NADPH/NADP(+) ratio in response to KIC were largely blunted in BCATm(-/-) islets. Compared with nontreated islets, the combination of KIC/glutamine (10/2 mM) did not influence α-ketoglutarate concentrations but caused 120 and 33% increases in malate in BCATm(+/+) and BCATm(-/-) islets, respectively. Although leucine oxidation and KIC transamination were blocked in BCATm(-/-) islets, KIC oxidation was unaltered. These data indicate that KICSIS requires transamination of KIC and glutamate to leucine and α-ketoglutarate, respectively. LSIS does not require leucine catabolism and may be through leucine activation of glutamate dehydrogenase. Thus, KICSIS and LSIS occur by enhancing the metabolism of glutamine/glutamate to α-ketoglutarate, which, in turn, is metabolized to produce the intracellular signals such as ATP and NADPH for insulin secretion.
Figures
References
-
- Henquin J. C., Dufrane D., Nenquin M. (2006) Diabetes 55, 3470–3477 - PubMed
-
- Hutton J. C., Sener A., Malaisse W. J. (1980) J. Biol. Chem. 255, 7340–7346 - PubMed
-
- Lenzen S., Formanek H., Panten U. (1982) J. Biol. Chem. 257, 6631–6633 - PubMed
-
- Malaisse W. J. (1986) Adv. Enzyme Regul. 25, 203–217 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

