Overoxidation of 2-Cys peroxiredoxin in prokaryotes: cyanobacterial 2-Cys peroxiredoxins sensitive to oxidative stress
- PMID: 20736168
- PMCID: PMC2966063
- DOI: 10.1074/jbc.M110.160465
Overoxidation of 2-Cys peroxiredoxin in prokaryotes: cyanobacterial 2-Cys peroxiredoxins sensitive to oxidative stress
Abstract
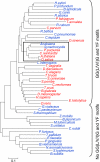
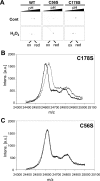
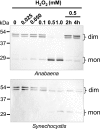
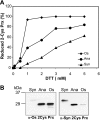
In eukaryotic organisms, hydrogen peroxide has a dual effect; it is potentially toxic for the cell but also has an important signaling activity. According to the previously proposed floodgate hypothesis, the signaling activity of hydrogen peroxide in eukaryotes requires a transient increase in its concentration, which is due to the inactivation by overoxidation of 2-Cys peroxiredoxin (2-Cys Prx). Sensitivity to overoxidation depends on the structural GGLG and YF motifs present in eukaryotic 2-Cys Prxs and is believed to be absent from prokaryotic enzymes, thus representing a paradoxical gain of function exclusive to eukaryotic organisms. Here we show that 2-Cys Prxs from several prokaryotic organisms, including cyanobacteria, contain the GG(L/V/I)G and YF motifs characteristic of sensitive enzymes. In search of the existence of overoxidation-sensitive 2-Cys Prxs in prokaryotes, we have analyzed the sensitivity to overoxidation of 2-Cys Prxs from two cyanobacterial strains, Anabaena sp. PCC7120 and Synechocystis sp. PCC6803. In vitro analysis of wild type and mutant variants of the Anabaena 2-Cys Prx showed that this enzyme is overoxidized at the peroxidatic cysteine residue, thus constituting an exception among prokaryotes. Moreover, the 2-Cys Prx from Anabaena is readily and reversibly overoxidized in vivo in response to high light and hydrogen peroxide, showing higher sensitivity to overoxidation than the Synechocystis enzyme. These cyanobacterial strains have different strategies to cope with hydrogen peroxide. While Synechocystis has low content of less sensitive 2-Cys Prx and high catalase activity, Anabaena contains abundant and sensitive 2-Cys Prx, but low catalase activity, which is remarkably similar to the chloroplast system.
Figures


Similar articles
-
Redox-dependent chaperone/peroxidase function of 2-Cys-Prx from the cyanobacterium Anabaena PCC7120: role in oxidative stress tolerance.BMC Plant Biol. 2015 Feb 21;15:60. doi: 10.1186/s12870-015-0444-2. BMC Plant Biol. 2015. PMID: 25849452 Free PMC article.
-
The contribution of NADPH thioredoxin reductase C (NTRC) and sulfiredoxin to 2-Cys peroxiredoxin overoxidation in Arabidopsis thaliana chloroplasts.J Exp Bot. 2015 May;66(10):2957-66. doi: 10.1093/jxb/eru512. Epub 2015 Jan 5. J Exp Bot. 2015. PMID: 25560178 Free PMC article.
-
A eukaryotic-like sulfiredoxin involved in oxidative stress responses and in the reduction of the sulfinic form of 2-Cys peroxiredoxin in the cyanobacterium Anabaena PCC 7120.New Phytol. 2011 Sep;191(4):1108-1118. doi: 10.1111/j.1469-8137.2011.03774.x. Epub 2011 Jun 8. New Phytol. 2011. PMID: 21651559
-
Multiple functions of 2-Cys peroxiredoxins, I and II, and their regulations via post-translational modifications.Free Radic Biol Med. 2020 May 20;152:107-115. doi: 10.1016/j.freeradbiomed.2020.02.028. Epub 2020 Mar 7. Free Radic Biol Med. 2020. PMID: 32151745 Review.
-
The Peroxiredoxin Family: An Unfolding Story.Subcell Biochem. 2017;83:127-147. doi: 10.1007/978-3-319-46503-6_5. Subcell Biochem. 2017. PMID: 28271475 Review.
Cited by
-
Expression of OsTPX Gene Improves Cellular Redox Homeostasis and Photosynthesis Efficiency in Synechococcus elongatus PCC 7942.Front Plant Sci. 2018 Dec 11;9:1848. doi: 10.3389/fpls.2018.01848. eCollection 2018. Front Plant Sci. 2018. PMID: 30619416 Free PMC article.
-
Overoxidation of chloroplast 2-Cys peroxiredoxins: balancing toxic and signaling activities of hydrogen peroxide.Front Plant Sci. 2013 Aug 19;4:310. doi: 10.3389/fpls.2013.00310. eCollection 2013. Front Plant Sci. 2013. PMID: 23967002 Free PMC article.
-
Cysteine-mediated redox signaling: chemistry, biology, and tools for discovery.Chem Rev. 2013 Jul 10;113(7):4633-79. doi: 10.1021/cr300163e. Epub 2013 Mar 20. Chem Rev. 2013. PMID: 23514336 Free PMC article. Review. No abstract available.
-
Redox regulation of chloroplast metabolism.Plant Physiol. 2021 May 27;186(1):9-21. doi: 10.1093/plphys/kiaa062. Plant Physiol. 2021. PMID: 33793865 Free PMC article. Review.
-
Redox-dependent chaperone/peroxidase function of 2-Cys-Prx from the cyanobacterium Anabaena PCC7120: role in oxidative stress tolerance.BMC Plant Biol. 2015 Feb 21;15:60. doi: 10.1186/s12870-015-0444-2. BMC Plant Biol. 2015. PMID: 25849452 Free PMC article.
References
-
- Veal E. A., Day A. M., Morgan B. A. (2007) Mol. Cell. 26, 1–14 - PubMed
-
- Chae H. Z., Chung S. J., Rhee S. G. (1994) J. Biol. Chem. 269, 27670–27678 - PubMed
-
- Chae H. Z., Kim H. J., Kang S. W., Rhee S. G. (1999) Diabetes Res. Clin. Pract. 45, 101–112 - PubMed
-
- Poole L. B. (2005) Arch. Biochem. Biophys. 433, 240–254 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

