Metabolism of pentose sugars in the hyperthermophilic archaea Sulfolobus solfataricus and Sulfolobus acidocaldarius
- PMID: 20736170
- PMCID: PMC2962468
- DOI: 10.1074/jbc.M110.146332
Metabolism of pentose sugars in the hyperthermophilic archaea Sulfolobus solfataricus and Sulfolobus acidocaldarius
Abstract
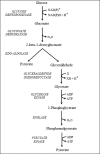
We have previously shown that the hyperthermophilic archaeon, Sulfolobus solfataricus, catabolizes d-glucose and d-galactose to pyruvate and glyceraldehyde via a non-phosphorylative version of the Entner-Doudoroff pathway. At each step, one enzyme is active with both C6 epimers, leading to a metabolically promiscuous pathway. On further investigation, the catalytic promiscuity of the first enzyme in this pathway, glucose dehydrogenase, has been shown to extend to the C5 sugars, D-xylose and L-arabinose. In the current paper we establish that this promiscuity for C6 and C5 metabolites is also exhibited by the third enzyme in the pathway, 2-keto-3-deoxygluconate aldolase, but that the second step requires a specific C5-dehydratase, the gluconate dehydratase being active only with C6 metabolites. The products of this pathway for the catabolism of D-xylose and L-arabinose are pyruvate and glycolaldehyde, pyruvate entering the citric acid cycle after oxidative decarboxylation to acetyl-coenzyme A. We have identified and characterized the enzymes, both native and recombinant, that catalyze the conversion of glycolaldehyde to glycolate and then to glyoxylate, which can enter the citric acid cycle via the action of malate synthase. Evidence is also presented that similar enzymes for this pentose sugar pathway are present in Sulfolobus acidocaldarius, and metabolic tracer studies in this archaeon demonstrate its in vivo operation in parallel with a route involving no aldol cleavage of the 2-keto-3-deoxy-pentanoates but direct conversion to the citric acid cycle C5-metabolite, 2-oxoglutarate.
Figures


Similar articles
-
Promiscuity in the part-phosphorylative Entner-Doudoroff pathway of the archaeon Sulfolobus solfataricus.FEBS Lett. 2005 Dec 19;579(30):6865-9. doi: 10.1016/j.febslet.2005.11.028. Epub 2005 Dec 1. FEBS Lett. 2005. PMID: 16330030
-
Metabolic pathway promiscuity in the archaeon Sulfolobus solfataricus revealed by studies on glucose dehydrogenase and 2-keto-3-deoxygluconate aldolase.J Biol Chem. 2003 Sep 5;278(36):34066-72. doi: 10.1074/jbc.M305818200. Epub 2003 Jun 24. J Biol Chem. 2003. PMID: 12824170
-
Sulfolobus acidocaldarius Transports Pentoses via a Carbohydrate Uptake Transporter 2 (CUT2)-Type ABC Transporter and Metabolizes Them through the Aldolase-Independent Weimberg Pathway.Appl Environ Microbiol. 2018 Jan 17;84(3):e01273-17. doi: 10.1128/AEM.01273-17. Print 2018 Feb 1. Appl Environ Microbiol. 2018. PMID: 29150511 Free PMC article.
-
Syntheses of 2-keto-3-deoxy-D-xylonate and 2-keto-3-deoxy-L-arabinonate as stereochemical probes for demonstrating the metabolic promiscuity of Sulfolobus solfataricus towards D-xylose and L-arabinose.Chemistry. 2013 Feb 18;19(8):2895-902. doi: 10.1002/chem.201203489. Epub 2013 Jan 11. Chemistry. 2013. PMID: 23315785
-
The chromosome replication machinery of the archaeon Sulfolobus solfataricus.J Biol Chem. 2006 Jun 2;281(22):15029-32. doi: 10.1074/jbc.R500029200. Epub 2006 Feb 8. J Biol Chem. 2006. PMID: 16467299 Review.
Cited by
-
Hot transcriptomics.Archaea. 2011 Feb 7;2010:897585. doi: 10.1155/2010/897585. Archaea. 2011. PMID: 21350598 Free PMC article.
-
Revealing oxidative pentose metabolism in new Pseudomonas putida isolates.Environ Microbiol. 2023 Feb;25(2):493-504. doi: 10.1111/1462-2920.16296. Epub 2022 Dec 11. Environ Microbiol. 2023. PMID: 36465038 Free PMC article.
-
Absence of diauxie during simultaneous utilization of glucose and Xylose by Sulfolobus acidocaldarius.J Bacteriol. 2011 Mar;193(6):1293-301. doi: 10.1128/JB.01219-10. Epub 2011 Jan 14. J Bacteriol. 2011. PMID: 21239580 Free PMC article.
-
Sulfolobus - A Potential Key Organism in Future Biotechnology.Front Microbiol. 2017 Dec 12;8:2474. doi: 10.3389/fmicb.2017.02474. eCollection 2017. Front Microbiol. 2017. PMID: 29312184 Free PMC article. Review.
-
Utilization of Phenol as Carbon Source by the Thermoacidophilic Archaeon Saccharolobus solfataricus P2 Is Limited by Oxygen Supply and the Cellular Stress Response.Front Microbiol. 2021 Jan 8;11:587032. doi: 10.3389/fmicb.2020.587032. eCollection 2020. Front Microbiol. 2021. PMID: 33488537 Free PMC article.
References
-
- Siebers B., Schönheit P. (2005) Curr. Opin. Microbiol. 8, 695–705 - PubMed
-
- Danson M. J., Lamble H. J., Hough D. W. (2007) Archaea: Molecular and Cellular Biology (Cavicchioli R. ed) pp. 260–287, American Society for Microbiology, Washington, DC
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

