Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial
- PMID: 20736298
- PMCID: PMC3228041
- DOI: 10.1634/theoncologist.2009-0181
Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial
Abstract
Objectives: A planned interim analysis of study EGF100151 prompted early termination of enrollment based on a longer time to progression with lapatinib and capecitabine than with capecitabine alone in patients with human epidermal growth factor receptor (HER)-2(+) previously treated advanced breast cancer or metastatic breast cancer (MBC). Here, we report final analyses of overall survival.
Patients and methods: Women with HER-2(+) MBC who progressed after regimens that included, but were not limited to, anthracyclines, taxanes, and trastuzumab, were randomized to lapatinib (1,250 mg/day) plus capecitabine (2,000 mg/m(2)) or capecitabine monotherapy (2,500 mg/m(2)) on days 1-14 of a 21-day cycle.
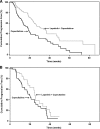
Results: At enrollment termination, 399 patients were randomized, and nine were being screened and were offered combination treatment. In total, 207 and 201 patients were enrolled to combination therapy and monotherapy, respectively. Thirty-six patients receiving monotherapy crossed over to combination therapy following enrollment termination. The median overall survival times were 75.0 weeks for the combination arm and 64.7 weeks for the monotherapy arm (hazard ratio [HR], 0.87; 95% confidence interval [CI], 0.71-1.08; p = .210). A Cox regression analysis considering crossover as a time-dependent covariate suggested a 20% lower risk for death for patients treated with combination therapy (HR, 0.80; 95% CI, 0.64-0.99; p = .043). The low incidence of serious adverse events was consistent with previously reported rates.
Conclusions: Although premature enrollment termination and subsequent crossover resulted in insufficient power to detect differences in overall survival, exploratory analyses demonstrate a trend toward a survival advantage with lapatinib plus capecitabine. These data continue to support the efficacy of lapatinib in patients with HER-2(+) MBC.
Conflict of interest statement
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers.
Figures



References
-
- Moy B, Goss PE. Lapatinib: Current status and future directions in breast cancer. The Oncologist. 2006;11:1047–1057. - PubMed
-
- Joensuu H, Kellokumpu-Lehtinen P-L, Bono P, et al. FinHer Study Investigators. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. - PubMed
-
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. - PubMed
-
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. - PubMed
-
- Slamon D, Eiermann W, Robert N, et al. BCIRG 006 Investigators. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC→T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2 positive early breast cancer patients: BCIRG 006 study [abstract 1] Breast Cancer Res Treat. 2005;94(suppl 1):S5.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

