Chemokine expression and control of muscle cell migration during myogenesis
- PMID: 20736301
- PMCID: PMC2931603
- DOI: 10.1242/jcs.066241
Chemokine expression and control of muscle cell migration during myogenesis
Abstract
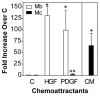
Adult regenerative myogenesis is vital for restoring normal tissue structure after muscle injury. Muscle regeneration is dependent on progenitor satellite cells, which proliferate in response to injury, and their progeny differentiate and undergo cell-cell fusion to form regenerating myofibers. Myogenic progenitor cells must be precisely regulated and positioned for proper cell fusion to occur. Chemokines are secreted proteins that share both leukocyte chemoattractant and cytokine-like behavior and affect the physiology of a number of cell types. We investigated the steady-state mRNA levels of 84 chemokines, chemokine receptors and signaling molecules, to obtain a comprehensive view of chemokine expression by muscle cells during myogenesis in vitro. A large number of chemokines and chemokine receptors were expressed by primary mouse muscle cells, especially during times of extensive cell-cell fusion. Furthermore, muscle cells exhibited different migratory behavior throughout myogenesis in vitro. One receptor-ligand pair, CXCR4-SDF-1alpha (CXCL12), regulated migration of both proliferating and terminally differentiated muscle cells, and was necessary for proper fusion of muscle cells. Given the large number of chemokines and chemokine receptors directly expressed by muscle cells, these proteins might have a greater role in myogenesis than previously appreciated.
Figures



Similar articles
-
Expression and function of the SDF-1 chemokine receptors CXCR4 and CXCR7 during mouse limb muscle development and regeneration.Exp Cell Res. 2012 Oct 15;318(17):2178-90. doi: 10.1016/j.yexcr.2012.06.020. Epub 2012 Jul 3. Exp Cell Res. 2012. PMID: 22766125
-
The effect of delivering the chemokine SDF-1α in a matrix-bound manner on myogenesis.Biomaterials. 2014 May;35(15):4525-4535. doi: 10.1016/j.biomaterials.2014.02.008. Epub 2014 Mar 5. Biomaterials. 2014. PMID: 24612919 Free PMC article.
-
Effect of myostatin on chemokine expression in regenerating skeletal muscle cells.Cells Tissues Organs. 2013;198(1):66-74. doi: 10.1159/000351462. Epub 2013 Jul 3. Cells Tissues Organs. 2013. PMID: 23838214
-
A guide to chemokines and their receptors.FEBS J. 2018 Aug;285(16):2944-2971. doi: 10.1111/febs.14466. Epub 2018 Apr 24. FEBS J. 2018. PMID: 29637711 Free PMC article. Review.
-
Chemokine-chemokine receptor network in immune cell trafficking.Curr Drug Targets Immune Endocr Metabol Disord. 2004 Dec;4(4):343-61. doi: 10.2174/1568008043339712. Curr Drug Targets Immune Endocr Metabol Disord. 2004. PMID: 15578986 Review.
Cited by
-
Distinct transcriptomic profile of satellite cells contributes to preservation of neuromuscular junctions in extraocular muscles of ALS mice.Elife. 2024 Apr 25;12:RP92644. doi: 10.7554/eLife.92644. Elife. 2024. PMID: 38661532 Free PMC article.
-
Cxcl14 depletion accelerates skeletal myogenesis by promoting cell cycle withdrawal.NPJ Regen Med. 2017;2:16017-. doi: 10.1038/npjregenmed.2016.17. Epub 2017 Jan 5. NPJ Regen Med. 2017. PMID: 28775895 Free PMC article.
-
Identification of CXCL11 as part of chemokine network controlling skeletal muscle development.Cell Tissue Res. 2021 May;384(2):499-511. doi: 10.1007/s00441-020-03398-0. Epub 2021 Jan 27. Cell Tissue Res. 2021. PMID: 33502606 Free PMC article.
-
cAMP signaling in skeletal muscle adaptation: hypertrophy, metabolism, and regeneration.Am J Physiol Endocrinol Metab. 2012 Jul 1;303(1):E1-17. doi: 10.1152/ajpendo.00555.2011. Epub 2012 Feb 21. Am J Physiol Endocrinol Metab. 2012. PMID: 22354781 Free PMC article. Review.
-
DUX4 activates germline genes, retroelements, and immune mediators: implications for facioscapulohumeral dystrophy.Dev Cell. 2012 Jan 17;22(1):38-51. doi: 10.1016/j.devcel.2011.11.013. Epub 2011 Dec 29. Dev Cell. 2012. PMID: 22209328 Free PMC article.
References
-
- Allbrook D. (1981). Skeletal muscle regeneration. Muscle Nerve 4, 234-245 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

