Formation of extra centrosomal structures is dependent on beta-catenin
- PMID: 20736306
- PMCID: PMC2931606
- DOI: 10.1242/jcs.064782
Formation of extra centrosomal structures is dependent on beta-catenin
Abstract
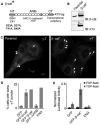
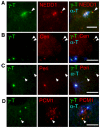
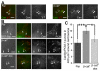
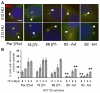
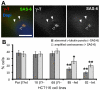
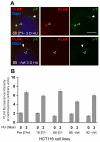
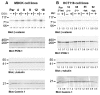
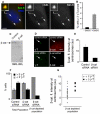
beta-Catenin has important roles in cell-cell adhesion and in the regulation of gene transcription. Mutations that stabilize beta-catenin are common in cancer, but it remains unclear how these mutations contribute to cancer progression. beta-Catenin is also a centrosomal component involved in centrosome separation. Centrosomes nucleate interphase microtubules and the bipolar mitotic spindle in normal cells, but their organization and function in human cancers are abnormal. Here, we show that expression of stabilized mutant beta-catenin, which mimics mutations found in cancer, results in extra non-microtubule nucleating structures that contain a subset of centrosome proteins including gamma-tubulin and centrin, but not polo-like kinase 4 (Plk4), SAS-6 or pericentrin. A transcriptionally inactive form of beta-catenin also gives rise to abnormal structures of centrosome proteins. HCT116 human colon cancer cell lines, from which the mutant beta-catenin allele has been deleted, have reduced numbers of cells with abnormal centrosome structures and S-phase-arrested, amplified centrosomes. RNAi-mediated depletion of beta-catenin from centrosomes inhibits S-phase-arrested amplification of centrosomes. These results indicate that beta-catenin is required for centrosome amplification, and mutations in beta-catenin might contribute to the formation of abnormal centrosomes observed in cancers.
Figures

Similar articles
-
beta-Catenin is a Nek2 substrate involved in centrosome separation.Genes Dev. 2008 Jan 1;22(1):91-105. doi: 10.1101/gad.1596308. Epub 2007 Dec 17. Genes Dev. 2008. PMID: 18086858 Free PMC article.
-
Phosphorylated Tyr142 β-catenin localizes to centrosomes and is regulated by Syk.J Cell Biochem. 2018 Apr;119(4):3632-3640. doi: 10.1002/jcb.26571. Epub 2018 Jan 5. J Cell Biochem. 2018. PMID: 29231998
-
Cep192 controls the balance of centrosome and non-centrosomal microtubules during interphase.PLoS One. 2014 Jun 27;9(6):e101001. doi: 10.1371/journal.pone.0101001. eCollection 2014. PLoS One. 2014. PMID: 24971877 Free PMC article.
-
β-catenin at the centrosome: discrete pools of β-catenin communicate during mitosis and may co-ordinate centrosome functions and cell cycle progression.Bioessays. 2013 Sep;35(9):804-9. doi: 10.1002/bies.201300045. Epub 2013 Jun 27. Bioessays. 2013. PMID: 23804296 Free PMC article. Review.
-
Centrosome function in normal and tumor cells.J Cell Biochem. 2006 Dec 1;99(5):1240-50. doi: 10.1002/jcb.21003. J Cell Biochem. 2006. PMID: 16817224 Review.
Cited by
-
EB1 levels are elevated in ascorbic Acid (AA)-stimulated osteoblasts and mediate cell-cell adhesion-induced osteoblast differentiation.J Biol Chem. 2013 Jul 26;288(30):22096-110. doi: 10.1074/jbc.M113.481515. Epub 2013 Jun 5. J Biol Chem. 2013. PMID: 23740245 Free PMC article.
-
The role of microtubules in the regulation of epithelial junctions.Tissue Barriers. 2018;6(3):1539596. doi: 10.1080/21688370.2018.1539596. Epub 2018 Nov 5. Tissue Barriers. 2018. PMID: 30395792 Free PMC article. Review.
-
Serum inducible kinase is a positive regulator of cortical dendrite development and is required for BDNF-promoted dendritic arborization.Cell Res. 2012 Feb;22(2):387-98. doi: 10.1038/cr.2011.100. Epub 2011 Jun 21. Cell Res. 2012. PMID: 21691298 Free PMC article.
-
PEDF regulates plasticity of a novel lipid-MTOC axis in prostate cancer-associated fibroblasts.J Cell Sci. 2018 Jul 11;131(13):jcs213579. doi: 10.1242/jcs.213579. J Cell Sci. 2018. PMID: 29792311 Free PMC article.
-
MicroRNA‑155‑5p inhibits trophoblast cell proliferation and invasion by disrupting centrosomal function.Mol Med Rep. 2024 May;29(5):85. doi: 10.3892/mmr.2024.13209. Epub 2024 Mar 29. Mol Med Rep. 2024. PMID: 38551159 Free PMC article.
References
-
- Aldaz H., Rice L. M., Stearns T., Agard D. A. (2005). Insights into microtubule nucleation from the crystal structure of human gamma-tubulin. Nature 435, 523-527 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

