The Chinese herbal medicine Sophora flavescens activates pregnane X receptor
- PMID: 20736322
- PMCID: PMC2993459
- DOI: 10.1124/dmd.110.035253
The Chinese herbal medicine Sophora flavescens activates pregnane X receptor
Abstract
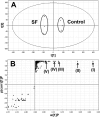
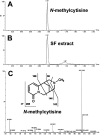
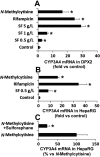
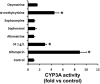
Sophora flavescens (SF) is an herbal medicine widely used for the treatment of viral hepatitis, cancer, viral myocarditis, gastrointestinal hemorrhage, and skin diseases. It was recently reported that SF up-regulates CYP3A expression. The mechanism of SF-induced CYP3A expression is unknown. In the current study, we tested the hypothesis that SF-induced CYP3A expression is mediated by the activation of pregnane X receptor (PXR). We used two cell lines, DPX2 and HepaRG, to investigate the role of PXR in SF-induced CYP3A expression. The DPX2 cell line is derived from HepG2 cells with the stable transfection of human PXR and a luciferase reporter gene linked with a human PXR response element identified in the CYP3A4 gene promoter. In DPX2 cells, SF activated PXR in a concentration-dependent manner. We used a metabolomic approach to identify the chemical constituents in SF, which were further analyzed for their effect on PXR activation and CYP3A regulation. One chemical in SF, N-methylcytisine, was identified as an individual chemical that activated PXR. HepaRG is a highly differentiated hepatoma cell line that mimics human hepatocytes. In HepaRG cells, N-methylcytisine significantly induced CYP3A4 expression, and this induction was suppressed by the PXR antagonist sulforaphane. These results suggest that SF induces CYP3A expression via the activation of PXR.
Figures
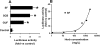
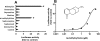
Similar articles
-
PXR-mediated transcriptional activation of CYP3A4 by cryptotanshinone and tanshinone IIA.Chem Biol Interact. 2009 Jan 15;177(1):58-64. doi: 10.1016/j.cbi.2008.08.013. Epub 2008 Sep 2. Chem Biol Interact. 2009. PMID: 18805405
-
Pregnane X receptor mediated-transcription regulation of CYP3A by glycyrrhizin: a possible mechanism for its hepatoprotective property against lithocholic acid-induced injury.Chem Biol Interact. 2012 Oct 25;200(1):11-20. doi: 10.1016/j.cbi.2012.08.023. Epub 2012 Sep 13. Chem Biol Interact. 2012. PMID: 22982774
-
Induction of human cytochrome P450 3A4 by the irreversible myeloperoxidase inactivator PF-06282999 is mediated by the pregnane X receptor.Xenobiotica. 2018 Jul;48(7):647-655. doi: 10.1080/00498254.2017.1353163. Epub 2017 Jul 24. Xenobiotica. 2018. PMID: 28685622
-
Pregnane X receptor: molecular basis for species differences in CYP3A induction by xenobiotics.Chem Biol Interact. 2001 May 16;134(3):283-9. doi: 10.1016/s0009-2797(01)00163-6. Chem Biol Interact. 2001. PMID: 11336976 Review.
-
Phosphorylation and protein-protein interactions in PXR-mediated CYP3A repression.Expert Opin Drug Metab Toxicol. 2009 Aug;5(8):861-73. doi: 10.1517/17425250903012360. Expert Opin Drug Metab Toxicol. 2009. PMID: 19505191 Free PMC article. Review.
Cited by
-
Impact of the herbal medicine Sophora flavescens on the oral pharmacokinetics of indinavir in rats: the involvement of CYP3A and P-glycoprotein.PLoS One. 2012;7(2):e31312. doi: 10.1371/journal.pone.0031312. Epub 2012 Feb 16. PLoS One. 2012. PMID: 22359586 Free PMC article.
-
Anti-Itching and Anti-Inflammatory Effects of Kushenol F via the Inhibition of TSLP Production.Pharmaceuticals (Basel). 2022 Oct 31;15(11):1347. doi: 10.3390/ph15111347. Pharmaceuticals (Basel). 2022. PMID: 36355519 Free PMC article.
-
A Systematic Review of Drug Metabolism Studies of Plants With Anticancer Properties: Approaches Applied and Limitations.Eur J Drug Metab Pharmacokinet. 2020 Apr;45(2):173-225. doi: 10.1007/s13318-019-00582-8. Eur J Drug Metab Pharmacokinet. 2020. PMID: 31679146
-
Andrographis paniculata Extract and Andrographolide Modulate the Hepatic Drug Metabolism System and Plasma Tolbutamide Concentrations in Rats.Evid Based Complement Alternat Med. 2013;2013:982689. doi: 10.1155/2013/982689. Epub 2013 Aug 12. Evid Based Complement Alternat Med. 2013. PMID: 23997806 Free PMC article.
-
Diversity patterns of soil microbial communities in the Sophora flavescens rhizosphere in response to continuous monocropping.BMC Microbiol. 2020 Aug 31;20(1):272. doi: 10.1186/s12866-020-01956-8. BMC Microbiol. 2020. PMID: 32867674 Free PMC article.
References
-
- Anthérieu S, Chesné C, Li R, Camus S, Lahoz A, Picazo L, Turpeinen M, Tolonen A, Uusitalo J, Guguen-Guillouzo C, et al. (2010) Stable expression, activity, and inducibility of cytochromes P450 in differentiated HepaRG cells. Drug Metab Dispos 38:516–525 - PubMed
-
- Chan E, Tan M, Xin J, Sudarsanam S, Johnson DE. (2010) Interactions between traditional Chinese medicines and Western therapeutics. Curr Opin Drug Discov Devel 13:50–65 - PubMed
-
- Chen C, Guo SM, Liu B. (2000) A randomized controlled trial of kurorinone versus interferon-α2a treatment in patients with chronic hepatitis B. J Viral Hepat 7:225–229 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

