Inconsistent labeling of food effect for oral agents across therapeutic areas: differences between oncology and non-oncology products
- PMID: 20736327
- PMCID: PMC2932769
- DOI: 10.1158/1078-0432.CCR-10-0663
Inconsistent labeling of food effect for oral agents across therapeutic areas: differences between oncology and non-oncology products
Abstract
Purpose: Several recent oral oncology drugs were labeled for administration in fasted states despite the fact that food increases their bioavailability. Because this was inconsistent with the principles of oral drug delivery, we hypothesized that there were inconsistencies across therapeutic areas.
Experimental design: Oral agents approved by the U.S. Food and Drug Administration from January 2000 to May 2009 were included in our study. Comparison of the food labeling patterns between oncology and non-oncology drugs was made using Fisher's exact test.
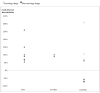
Results: Of the 99 drugs evaluated, 34 showed significant food effects on bioavailability. When food markedly enhanced bioavailability, eight out of nine non-oncology drugs were labeled "fed" to take advantage of the food-drug interaction, whereas all oncology drugs (n = 3) were labeled to be administered in "fasted" states (Fisher's exact test, P = 0.01).
Conclusions: Drug labeling patterns with respect to food-drug interactions observed with oncology drugs are in contradiction with fundamental pharmacologic principles, as exemplified in the labeling of non-oncology drugs. .
Figures
Comment in
-
Food and oral antineoplastics: more than meets the eye.Clin Cancer Res. 2010 Sep 1;16(17):4305-7. doi: 10.1158/1078-0432.CCR-10-1857. Epub 2010 Aug 24. Clin Cancer Res. 2010. PMID: 20736331
References
-
- DeMario MD, Ratain MJ. Oral chemotherapy: rationale and future directions. J Clin Oncol. 1998 Jul;16(7):2557–67. - PubMed
-
- Partridge AH, Avorn J, Wang PS, Winer EP. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002 May 1;94(9):652–61. - PubMed
-
- Singh BN, Malhotra BK. Effects of food on the clinical pharmacokinetics of anticancer agents: underlying mechanisms and implications for oral chemotherapy. Clin Pharmacokinet. 2004;43(15):1127–56. - PubMed
-
- Ratain MJ, Cohen EE. The value meal: how to save $1,700 per month or more on lapatinib. J Clin Oncol. 2007 Aug 10;25(23):3397–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

