Substitution of adenovirus serotype 3 hexon onto a serotype 5 oncolytic adenovirus reduces factor X binding, decreases liver tropism, and improves antitumor efficacy
- PMID: 20736345
- PMCID: PMC2945233
- DOI: 10.1158/1535-7163.MCT-10-0332
Substitution of adenovirus serotype 3 hexon onto a serotype 5 oncolytic adenovirus reduces factor X binding, decreases liver tropism, and improves antitumor efficacy
Abstract
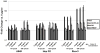
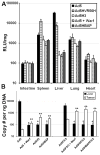
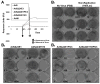
Following intravascular delivery, an important route of administration for many clinical applications, the liver is the predominant site of adenovirus serotype 5 (Ad5) sequestration, thereby posing a risk of toxicity. In this regard, it has recently been shown that the Ad5 capsid binds to the blood coagulation factor X (FX) via the Ad5 hexon protein. This interaction mediates the majority of Ad5 liver transduction. Patient FX levels can be diminished by the administration of warfarin, a vitamin K inhibitor in the liver that decreases FX production; however, warfarin is a potent anticoagulant and can have a number of undesired side effects. Therefore, genetic modification of the virus to ablate FX binding is the preferred approach. Modifications of the hexon protein, specifically within the hypervariable 5 (HVR5) and 7 (HVR7) regions, have produced Ad5 vectors that show minimal liver sequestration. Our laboratory has pioneered adenovirus hexon modifications, including insertion of peptide ligands into the hypervariable regions and substitution of the adenovirus hexon with hexon proteins from alternate serotypes. Substitution of the adenovirus serotype 3 (Ad3) hexon protein onto the Ad5 capsid has been further characterized with regard to its interaction with FX and incorporated into an infectivity-enhanced conditionally replicative adenovirus (CRAd). In vitro evaluation of these hexon-modified vectors showed decreased binding to FX and decreased cell transduction via FX-mediated pathways. Furthermore, in vivo biodistribution studies in mice exhibited a decrease in liver sequestration. With the use of xenograft tumor models, the antitumor efficacy of the hexon-modified CRAds was enhanced over nonmodified controls.
Conflict of interest statement
Conflicts of interest: none
Figures

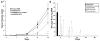
References
-
- Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2007--an update. J Gene Med. 2007;9:833–42. - PubMed
-
- Imperiale MJ. Keeping adenovirus away from the liver. Cell Host Microbe. 2008;3:119–20. - PubMed
-
- Parker AL, Waddington SN, Nicol CG, et al. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108:2554–61. - PubMed
-
- Waddington SN, McVey JH, Bhella D, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

