Altered distributions of Gemini of coiled bodies and mitochondria in motor neurons of TDP-43 transgenic mice
- PMID: 20736350
- PMCID: PMC2941282
- DOI: 10.1073/pnas.1003459107
Altered distributions of Gemini of coiled bodies and mitochondria in motor neurons of TDP-43 transgenic mice
Abstract
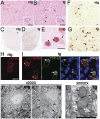
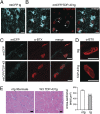
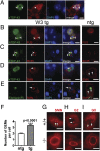
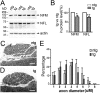
TAR DNA-binding protein-43 (TDP-43), a DNA/RNA-binding protein involved in RNA transcription and splicing, has been associated with the pathophysiology of neurodegenerative diseases, including ALS. However, the function of TDP-43 in motor neurons remains undefined. Here we use both gain- and loss-of-function approaches to determine roles of TDP-43 in motor neurons. Mice expressing human TDP-43 in neurons exhibited growth retardation and premature death that are characterized by abnormal intranuclear inclusions composed of TDP-43 and fused in sarcoma/translocated in liposarcoma (FUS/TLS), and massive accumulation of mitochondria in TDP-43-negative cytoplasmic inclusions in motor neurons, lack of mitochondria in motor axon terminals, and immature neuromuscular junctions. Whereas an elevated level of TDP-43 disrupts the normal nuclear distribution of survival motor neuron (SMN)-associated Gemini of coiled bodies (GEMs) in motor neurons, its absence prevents the formation of GEMs in the nuclei of these cells. Moreover, transcriptome-wide deep sequencing analysis revealed that a decrease in abundance of neurofilament transcripts contributed to the reduction of caliber of motor axons in TDP-43 mice. In concert, our findings indicate that TDP-43 participates in pathways critical for motor neuron physiology, including those that regulate the normal distributions of SMN-associated GEMs in the nucleus and mitochondria in the cytoplasm.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Decreased number of Gemini of coiled bodies and U12 snRNA level in amyotrophic lateral sclerosis.Hum Mol Genet. 2013 Oct 15;22(20):4136-47. doi: 10.1093/hmg/ddt262. Epub 2013 Jun 4. Hum Mol Genet. 2013. PMID: 23740936
-
TDP-43 expression in mouse models of amyotrophic lateral sclerosis and spinal muscular atrophy.BMC Neurosci. 2008 Oct 28;9:104. doi: 10.1186/1471-2202-9-104. BMC Neurosci. 2008. PMID: 18957104 Free PMC article.
-
Wild-type human TDP-43 expression causes TDP-43 phosphorylation, mitochondrial aggregation, motor deficits, and early mortality in transgenic mice.J Neurosci. 2010 Aug 11;30(32):10851-9. doi: 10.1523/JNEUROSCI.1630-10.2010. J Neurosci. 2010. PMID: 20702714 Free PMC article.
-
Minor splicing pathway is not minor any more: implications for the pathogenesis of motor neuron diseases.Neuropathology. 2014 Feb;34(1):99-107. doi: 10.1111/neup.12070. Epub 2013 Sep 22. Neuropathology. 2014. PMID: 24112438 Review.
-
The molecular link between inefficient GluA2 Q/R site-RNA editing and TDP-43 pathology in motor neurons of sporadic amyotrophic lateral sclerosis patients.Brain Res. 2014 Oct 10;1584:28-38. doi: 10.1016/j.brainres.2013.12.011. Epub 2013 Dec 16. Brain Res. 2014. PMID: 24355598 Review.
Cited by
-
Quantification of the Relative Contributions of Loss-of-function and Gain-of-function Mechanisms in TAR DNA-binding Protein 43 (TDP-43) Proteinopathies.J Biol Chem. 2016 Sep 9;291(37):19437-48. doi: 10.1074/jbc.M116.737726. Epub 2016 Jul 21. J Biol Chem. 2016. PMID: 27445339 Free PMC article.
-
The overexpression of TDP-43 in astrocytes causes neurodegeneration via a PTP1B-mediated inflammatory response.J Neuroinflammation. 2020 Oct 14;17(1):299. doi: 10.1186/s12974-020-01963-6. J Neuroinflammation. 2020. PMID: 33054766 Free PMC article.
-
The wobbler mouse, an ALS animal model.Mol Genet Genomics. 2013 Jun;288(5-6):207-29. doi: 10.1007/s00438-013-0741-0. Epub 2013 Mar 29. Mol Genet Genomics. 2013. PMID: 23539154 Free PMC article. Review.
-
Axonal transport deficits and degeneration can evolve independently in mouse models of amyotrophic lateral sclerosis.Proc Natl Acad Sci U S A. 2012 Mar 13;109(11):4296-301. doi: 10.1073/pnas.1200658109. Epub 2012 Feb 27. Proc Natl Acad Sci U S A. 2012. PMID: 22371592 Free PMC article.
-
Accelerated disease onset with stabilized familial amyotrophic lateral sclerosis (ALS)-linked mutant TDP-43 proteins.J Biol Chem. 2013 Feb 1;288(5):3641-54. doi: 10.1074/jbc.M112.433615. Epub 2012 Dec 12. J Biol Chem. 2013. PMID: 23235148 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

