Intravascular pressure augments cerebral arterial constriction by inducing voltage-insensitive Ca2+ waves
- PMID: 20736418
- PMCID: PMC3000587
- DOI: 10.1113/jphysiol.2010.193300
Intravascular pressure augments cerebral arterial constriction by inducing voltage-insensitive Ca2+ waves
Abstract
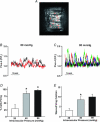
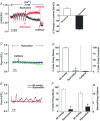
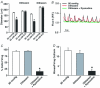
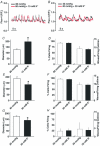
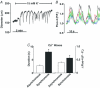
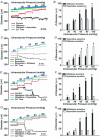
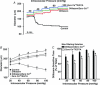
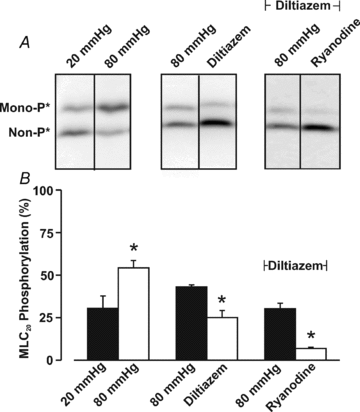
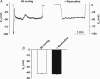
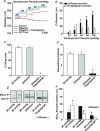
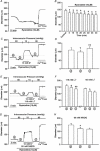
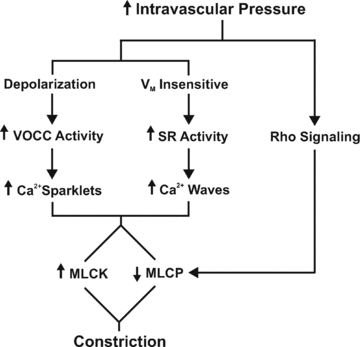
This study examined whether elevated intravascular pressure stimulates asynchronous Ca(2+) waves in cerebral arterial smooth muscle cells and if their generation contributes to myogenic tone development. The endothelium was removed from rat cerebral arteries, which were then mounted in an arteriograph, pressurized (20-100 mmHg) and examined under a variety of experimental conditions. Diameter and membrane potential (V(M)) were monitored using conventional techniques; Ca(2+) wave generation and myosin light chain (MLC(20))/MYPT1 (myosin phosphatase targeting subunit) phosphorylation were assessed by confocal microscopy and Western blot analysis, respectively. Elevating intravascular pressure increased the proportion of smooth muscle cells firing asynchronous Ca(2+) waves as well as event frequency. Ca(2+) wave augmentation occurred primarily at lower intravascular pressures (<60 mmHg) and ryanodine, a plant alkaloid that depletes the sarcoplasmic reticulum (SR) of Ca(2+), eliminated these events. Ca(2+) wave generation was voltage insensitive as Ca(2+) channel blockade and perturbations in extracellular [K(+)] had little effect on measured parameters. Ryanodine-induced inhibition of Ca(2+) waves attenuated myogenic tone and MLC(20) phosphorylation without altering arterial V(M). Thapsigargin, an SR Ca(2+)-ATPase inhibitor also attenuated Ca(2+) waves, pressure-induced constriction and MLC(20) phosphorylation. The SR-driven component of the myogenic response was proportionally greater at lower intravascular pressures and subsequent MYPT1 phosphorylation measures revealed that SR Ca(2+) waves facilitated pressure-induced MLC(20) phosphorylation through mechanisms that include myosin light chain phosphatase inhibition. Cumulatively, our findings show that mechanical stimuli augment Ca(2+) wave generation in arterial smooth muscle and that these transient events facilitate tone development particularly at lower intravascular pressures by providing a proportion of the Ca(2+) required to directly control MLC(20) phosphorylation.
Figures



Similar articles
-
Implications of αvβ3 Integrin Signaling in the Regulation of Ca2+ Waves and Myogenic Tone in Cerebral Arteries.Arterioscler Thromb Vasc Biol. 2015 Dec;35(12):2571-8. doi: 10.1161/ATVBAHA.115.305619. Epub 2015 Oct 22. Arterioscler Thromb Vasc Biol. 2015. PMID: 26494230
-
Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels.J Physiol. 1998 Apr 1;508 ( Pt 1)(Pt 1):211-21. doi: 10.1111/j.1469-7793.1998.211br.x. J Physiol. 1998. PMID: 9490841 Free PMC article.
-
Intravascular pressure regulates local and global Ca(2+) signaling in cerebral artery smooth muscle cells.Am J Physiol Cell Physiol. 2001 Aug;281(2):C439-48. doi: 10.1152/ajpcell.2001.281.2.C439. Am J Physiol Cell Physiol. 2001. PMID: 11443043
-
Cellular signalling in arteriolar myogenic constriction: involvement of tyrosine phosphorylation pathways.Clin Exp Pharmacol Physiol. 2002 Jul;29(7):612-9. doi: 10.1046/j.1440-1681.2002.03698.x. Clin Exp Pharmacol Physiol. 2002. PMID: 12060106 Review.
-
Ca2+ channels, ryanodine receptors and Ca(2+)-activated K+ channels: a functional unit for regulating arterial tone.Acta Physiol Scand. 1998 Dec;164(4):577-87. doi: 10.1046/j.1365-201X.1998.00462.x. Acta Physiol Scand. 1998. PMID: 9887980 Review.
Cited by
-
The role of actin filament dynamics in the myogenic response of cerebral resistance arteries.J Cereb Blood Flow Metab. 2013 Jan;33(1):1-12. doi: 10.1038/jcbfm.2012.144. Epub 2012 Oct 17. J Cereb Blood Flow Metab. 2013. PMID: 23072746 Free PMC article. Review.
-
Recruitment of dynamic endothelial Ca2+ signals by the TRPA1 channel activator AITC in rat cerebral arteries.Microcirculation. 2013 Feb;20(2):138-48. doi: 10.1111/micc.12004. Microcirculation. 2013. PMID: 22928941 Free PMC article.
-
Inositol 1,4,5-Trisphosphate Receptors in Hypertension.Front Physiol. 2018 Jul 26;9:1018. doi: 10.3389/fphys.2018.01018. eCollection 2018. Front Physiol. 2018. PMID: 30093868 Free PMC article. Review.
-
Electro-metabolic signaling.J Gen Physiol. 2024 Feb 5;156(2):e202313451. doi: 10.1085/jgp.202313451. Epub 2024 Jan 10. J Gen Physiol. 2024. PMID: 38197953 Free PMC article.
-
IP3 receptor orchestrates maladaptive vascular responses in heart failure.J Clin Invest. 2022 Feb 15;132(4):e152859. doi: 10.1172/JCI152859. J Clin Invest. 2022. PMID: 35166236 Free PMC article.
References
-
- Adb El-Rahman R, Turner R, Welsh DG. T- and L-type Ca2+ channels contribute to myogenic tone in cerebral arteries. FASEB J. 2010;24:1033.
-
- Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

