Protease-activated receptors mediate crosstalk between coagulation and fibrinolysis
- PMID: 20736455
- PMCID: PMC3012597
- DOI: 10.1182/blood-2010-06-293126
Protease-activated receptors mediate crosstalk between coagulation and fibrinolysis
Abstract
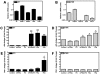
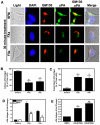
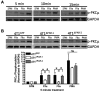
The coagulation and fibrinolytic systems contribute to malignancy by increasing angiogenesis, tumor growth, tumor invasion, and tumor metastasis. Oncogenic transformation increases the expression of tissue factor (TF) that results in local generation of coagulation proteases and activation of protease-activated receptor (PAR)-1 and PAR-2. We compared the PAR-dependent expression of urokinase plasminogen activator (uPA) and plasminogen activator inhibitor (PAI)-1 in 2 murine mammary adencocarcinoma cell lines: metastatic 4T1 cells and nonmetastatic 67NR cells. 4T1 cells expressed TF, PAR-1 and PAR-2 whereas 67NR cells expressed TF and PAR-1. We also silenced PAR-1 or PAR-2 expression in the 4T1 cells. We discovered 2 distinct mechanisms for PAR-dependent expression of uPA and PAI-1. First, we found that factor Xa or thrombin activation of PAR-1 led to a rapid release of stored intracellular uPA into the culture supernatant. Second, thrombin transactivation of a PAR-1/PAR-2 complex resulted in increases in PAI-1 mRNA and protein expression. Cells lacking PAR-2 failed to express PAI-1 in response to thrombin and factor Xa did not activate the PAR-1/PAR-2 complex. Our results reveal how PAR-1 and PAR-2 on tumor cells mediate crosstalk between coagulation and fibrinolysis.
Figures



References
-
- Magnus N, Garnier D, Rak J. Oncogenic epidermal growth factor receptor upregulates multiple elements of the tissue factor signaling pathway in human glioma cells. Blood. 2010;116(5):815–818. - PubMed
-
- Yu JL, May L, Lhotak V, et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105(4):1734–1741. - PubMed
-
- Hembrough TA, Swartz GM, Papathanassiu A, et al. Tissue factor/factor VIIa inhibitors block angiogenesis and tumor growth through a nonhemostatic mechanism. Cancer Res. 2003;63(11):2997–3000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

