Oct-3/4 regulates stem cell identity and cell fate decisions by modulating Wnt/β-catenin signalling
- PMID: 20736927
- PMCID: PMC2957205
- DOI: 10.1038/emboj.2010.200
Oct-3/4 regulates stem cell identity and cell fate decisions by modulating Wnt/β-catenin signalling
Abstract
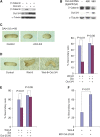
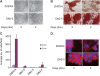
Although the transcriptional regulatory events triggered by Oct-3/4 are well documented, understanding the proteomic networks that mediate the diverse functions of this POU domain homeobox protein remains a major challenge. Here, we present genetic and biochemical studies that suggest an unexpected novel strategy for Oct-3/4-dependent regulation of embryogenesis and cell lineage determination. Our data suggest that Oct-3/4 specifically interacts with nuclear β-catenin and facilitates its proteasomal degradation, resulting in the maintenance of an undifferentiated, early embryonic phenotype both in Xenopus embryos and embryonic stem (ES) cells. Our data also show that Oct-3/4-mediated control of β-catenin stability has an important function in regulating ES cell motility. Down-regulation of Oct-3/4 increases β-catenin protein levels, enhancing Wnt signalling and initiating invasive cellular activity characteristic of epithelial-mesenchymal transition. Our data suggest a novel mode of regulation by which a delicate balance between β-catenin, Tcf3 and Oct-3/4 regulates maintenance of stem cell identity. Altering the balance between these proteins can direct cell fate decisions and differentiation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Aubert J, Dunstan H, Chambers I, Smith A (2002) Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat Biotechnol 20: 1240–1245 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

