Co-expression of CD147 (EMMPRIN), CD44v3-10, MDR1 and monocarboxylate transporters is associated with prostate cancer drug resistance and progression
- PMID: 20736947
- PMCID: PMC2965856
- DOI: 10.1038/sj.bjc.6605839
Co-expression of CD147 (EMMPRIN), CD44v3-10, MDR1 and monocarboxylate transporters is associated with prostate cancer drug resistance and progression
Abstract
Background: The aim of this study is to seek an association between markers of metastatic potential, drug resistance-related protein and monocarboxylate transporters in prostate cancer (CaP).
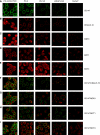
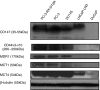
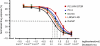
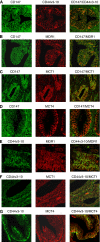
Methods: We evaluated the expression of invasive markers (CD147, CD44v3-10), drug-resistance protein (MDR1) and monocarboxylate transporters (MCT1 and MCT4) in CaP metastatic cell lines and CaP tissue microarrays (n=140) by immunostaining. The co-expression of CD147 and CD44v3-10 with that of MDR1, MCT1 and MCT4 in CaP cell lines was evaluated using confocal microscopy. The relationship between the expression of CD147 and CD44v3-10 and the sensitivity (IC(50)) to docetaxel in CaP cell lines was assessed using MTT assay. The relationship between expression of CD44v3-10, MDR1 and MCT4 and various clinicopathological CaP progression parameters was examined.
Results: CD147 and CD44v3-10 were co-expressed with MDR1, MCT1 and MCT4 in primary and metastatic CaP cells. Both CD147 and CD44v3-10 expression levels were inversely related to docetaxel sensitivity (IC(50)) in metastatic CaP cell lines. Overexpression of CD44v3-10, MDR1 and MCT4 was found in most primary CaP tissues, and was significantly associated with CaP progression.
Conclusions: Our results suggest that the overexpression of CD147, CD44v3-10, MDR1 and MCT4 is associated with CaP progression. Expression of both CD147 and CD44v3-10 is correlated with drug resistance during CaP metastasis and could be a useful potential therapeutic target in advanced disease.
Figures

References
-
- Albers MJ, Bok R, Chen AP, Cunningham CH, Zierhut ML, Zhang VY, Kohler SJ, Tropp J, Hurd RE, Yen YF, Nelson SJ, Vigneron DB, Kurhanewicz J (2008) Hyperpolarized 13C lactate, pyruvate, and alanine: noninvasive biomarkers for prostate cancer detection and grading. Cancer Res 68: 8607–8615 - PMC - PubMed
-
- Chen CT, Gan Y, Au JL, Wientjes MG (1998) Androgen-dependent and -independent human prostate xenograft tumours as models for drug activity evaluation. Cancer Res 58: 2777–2783 - PubMed
-
- Colone M, Calcabrini A, Toccacieli L, Bozzuto G, Stringaro A, Gentile M, Cianfriglia M, Ciervo A, Caraglia M, Budillon A, Meo G, Arancia G, Molinari A (2008) The multidrug transporter P-glycoprotein: a mediator of melanoma invasion? J Invest Dermatol 128: 957–971 - PubMed
-
- Cozzi PJ, Wang J, Delprado W, Madigan MC, Fairy S, Russell PJ, Li Y (2006) Evaluation of urokinase plasminogen activator and its receptor in different grades of human prostate cancer. Human Pathol 37: 1442–1451 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

