Combination of temozolomide with immunocytokine F16-IL2 for the treatment of glioblastoma
- PMID: 20736949
- PMCID: PMC2966626
- DOI: 10.1038/sj.bjc.6605832
Combination of temozolomide with immunocytokine F16-IL2 for the treatment of glioblastoma
Abstract
Background: Glioblastoma patients are still not cured by the treatments available at the moment. We investigated the therapeutic properties of temozolomide in combination with F16-IL2, a clinical-stage immunocytokine consisting of human interleukin (IL)-2 fused to the human antibody F16, specific to the A1 domain of tenascin-C.
Methods: We conducted three preclinical therapy studies, using subcutaneous and intracranial U87MG glioblastoma tumours xenografted in BALB/c nude mice. The same therapeutic schedule was used, consisting of five total administrations every third day, of 0.525 mg temozolomide, 20 microg F16-IL2, the combination, or the control solution.
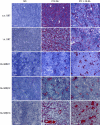
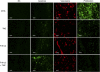
Results: Immunohistochemical analysis of U87MG xenografts and of human glioblastoma specimens showed selective tumour staining of F16. A quantitative biodistribution confirmed the preferential tumour accumulation of radiolabelled F16-IL2. In the study with subcutaneous xenografts, the combination of F16-IL2 with temozolomide induced complete remission of the animals, which remained tumour free for over 160 days. The same treatment led to a consistent size reduction of intracranial xenografts and to a longer survival of animals. The immunocytokine promoted the recruitment of leukocytes into tumours of both models.
Conclusion: The combined use of temozolomide with F16-IL2 deserves clinical investigations, which will be facilitated by the excellent safety profile in cynomolgus monkeys, and by the fact that F16-IL2 is in clinical trials in patients with cancer.
Figures



Similar articles
-
Antibody-mediated delivery of interleukin-2 to the stroma of breast cancer strongly enhances the potency of chemotherapy.Clin Cancer Res. 2008 Oct 15;14(20):6515-24. doi: 10.1158/1078-0432.CCR-07-5041. Clin Cancer Res. 2008. PMID: 18927291
-
Antitumor activity of (2E,5Z)-5-(2-hydroxybenzylidene)-2-((4-phenoxyphenyl)imino) thiazolidin-4-one, a novel microtubule-depolymerizing agent, in U87MG human glioblastoma cells and corresponding mouse xenograft model.J Pharmacol Sci. 2013;122(3):223-31. doi: 10.1254/jphs.13064fp. J Pharmacol Sci. 2013. PMID: 23877018
-
Convection-enhanced delivery of a synthetic retinoid Am80, loaded into polymeric micelles, prolongs the survival of rats bearing intracranial glioblastoma xenografts.Tohoku J Exp Med. 2010 Aug;221(4):257-64. doi: 10.1620/tjem.221.257. Tohoku J Exp Med. 2010. PMID: 20622491
-
Anticancer drug candidate CBL0137, which inhibits histone chaperone FACT, is efficacious in preclinical orthotopic models of temozolomide-responsive and -resistant glioblastoma.Neuro Oncol. 2017 Feb 1;19(2):186-196. doi: 10.1093/neuonc/now141. Neuro Oncol. 2017. PMID: 27370399 Free PMC article.
-
Temozolomide. A new option for high-grade astrocytomas.Cancer Pract. 2000 Nov-Dec;8(6):311-3. doi: 10.1046/j.1523-5394.2000.86004.x. Cancer Pract. 2000. PMID: 11898150 Review. No abstract available.
Cited by
-
TNC upregulation promotes glioma tumourigenesis through TDG-mediated active DNA demethylation.Cell Death Discov. 2024 Aug 1;10(1):347. doi: 10.1038/s41420-024-02098-w. Cell Death Discov. 2024. PMID: 39090080 Free PMC article.
-
Anti-cancer Therapies Employing IL-2 Cytokine Tumor Targeting: Contribution of Innate, Adaptive and Immunosuppressive Cells in the Anti-tumor Efficacy.Front Immunol. 2018 Dec 18;9:2905. doi: 10.3389/fimmu.2018.02905. eCollection 2018. Front Immunol. 2018. PMID: 30619269 Free PMC article. Review.
-
F8-IL10: A New Potential Antirheumatic Drug Evaluated by a PET-Guided Translational Approach.Mol Pharm. 2019 Jan 7;16(1):273-281. doi: 10.1021/acs.molpharmaceut.8b00982. Epub 2018 Dec 24. Mol Pharm. 2019. PMID: 30550295 Free PMC article.
-
Advances in tenascin-C biology.Cell Mol Life Sci. 2011 Oct;68(19):3175-99. doi: 10.1007/s00018-011-0783-6. Epub 2011 Aug 5. Cell Mol Life Sci. 2011. PMID: 21818551 Free PMC article. Review.
-
The antibody-mediated targeted delivery of interleukin-13 to syngeneic murine tumors mediates a potent anticancer activity.Cancer Immunol Immunother. 2015 May;64(5):635-44. doi: 10.1007/s00262-015-1666-8. Epub 2015 Feb 27. Cancer Immunol Immunother. 2015. PMID: 25722088 Free PMC article.
References
-
- Adams GP, Weiner LM (2005) Monoclonal antibody therapy of cancer. Nat Biotechnol 23: 1147–1157 - PubMed
-
- Albertsson PA, Basse PH, Hokland M, Goldfarb RH, Nagelkerke JF, Nannmark U, Kuppen PJ (2003) NK cells and the tumour microenvironment: implications for NK-cell function and anti-tumour activity. Trends Immunol 24: 603–609 - PubMed
-
- Balza E, Carnemolla B, Mortara L, Castellani P, Soncini D, Accolla RS, Borsi L (2010) Therapy-induced antitumor vaccination in neuroblastomas by the combined targeting of IL-2 and TNFalpha. Int J Cancer 127: 101–110 - PubMed
-
- Bello L, Lucini V, Costa F, Pluderi M, Giussani C, Acerbi F, Carrabba G, Pannacci M, Caronzolo D, Grosso S, Shinkaruk S, Colleoni F, Canron X, Tomei G, Deleris G, Bikfalvi A (2004) Combinatorial administration of molecules that simultaneously inhibit angiogenesis and invasion leads to increased therapeutic efficacy in mouse models of malignant glioma. Clin Cancer Res 10: 4527–4537 - PubMed
-
- Berndorff D, Borkowski S, Moosmayer D, Viti F, Müller-Tiemann B, Sieger S, Friebe M, Hilger CS, Zardi L, Neri D, Dinkelborg LM (2006) Imaging of tumor angiogenesis using 99mTc-labeled human recombinant anti-ED-B fibronectin antibody fragments. J Nucl Med 47: 1707–1716 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

