Simultaneous monitoring of tissue PO2 and NADH fluorescence during synaptic stimulation and spreading depression reveals a transient dissociation between oxygen utilization and mitochondrial redox state in rat hippocampal slices
- PMID: 20736960
- PMCID: PMC3049517
- DOI: 10.1038/jcbfm.2010.136
Simultaneous monitoring of tissue PO2 and NADH fluorescence during synaptic stimulation and spreading depression reveals a transient dissociation between oxygen utilization and mitochondrial redox state in rat hippocampal slices
Abstract
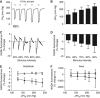
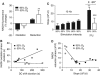
Nicotinamide adenine dinucleotide (NADH) imaging can be used to monitor neuronal activation and ascertain mitochondrial dysfunction, for example during hypoxia. During neuronal stimulation in vitro, NADH normally becomes more oxidized, indicating enhanced oxygen utilization. A subsequent NADH overshoot during activation or on recovery remains controversial and reflects either increased metabolic activity or limited oxygen availability. Tissue P(2) measurements, obtained simultaneously with NADH imaging in area CA1 in hippocampal slices, reveal that during prolonged train stimulation (ST) in 95% O(2), a persistent NADH oxidation is coupled with increased metabolic demand and oxygen utilization, for the duration of the stimulation. However, under conditions of either decreased oxygen supply (ST-50% O(2)) or enhanced metabolic demand (K(+)-induced spreading depression (K(+)-SD) 95% O(2)) the NADH oxidation is brief and the redox balance shifts early toward reduction, leading to a prolonged NADH overshoot. Yet, oxygen utilization remains elevated and is correlated with metabolic demand. Under these conditions, it appears that the rate of NAD(+) reduction may transiently exceed oxidation, to maintain an adequate oxygen flux and ATP production. In contrast, during SD in 50% O(2), the oxygen levels dropped to a point at which oxidative metabolism in the electron transport chain is limited and the rate of utilization declined.
Figures




Similar articles
-
Nicotinamide pre-treatment ameliorates NAD(H) hyperoxidation and improves neuronal function after severe hypoxia.Neurobiol Dis. 2014 Feb;62:469-78. doi: 10.1016/j.nbd.2013.10.025. Epub 2013 Oct 31. Neurobiol Dis. 2014. PMID: 24184921 Free PMC article.
-
Age-related metabolic fatigue during low glucose conditions in rat hippocampus.Neurobiol Aging. 2015 Feb;36(2):982-92. doi: 10.1016/j.neurobiolaging.2014.09.016. Epub 2014 Sep 28. Neurobiol Aging. 2015. PMID: 25443286 Free PMC article.
-
Interaction between tissue oxygen tension and NADH imaging during synaptic stimulation and hypoxia in rat hippocampal slices.Neuroscience. 2005;132(3):645-57. doi: 10.1016/j.neuroscience.2005.01.040. Neuroscience. 2005. PMID: 15837126
-
Differences in O2 availability resolve the apparent discrepancies in metabolic intrinsic optical signals in vivo and in vitro.Trends Neurosci. 2007 Aug;30(8):390-8. doi: 10.1016/j.tins.2007.06.001. Epub 2007 Jun 27. Trends Neurosci. 2007. PMID: 17590447 Free PMC article. Review.
-
Brain NADH redox state monitored in vivo by fiber optic surface fluorometry.Brain Res. 1984 Mar;319(1):49-68. doi: 10.1016/0165-0173(84)90029-8. Brain Res. 1984. PMID: 6370376 Review.
Cited by
-
Effect of temperature on FAD and NADH-derived signals and neurometabolic coupling in the mouse auditory and motor cortex.Pflugers Arch. 2017 Dec;469(12):1631-1649. doi: 10.1007/s00424-017-2037-4. Epub 2017 Aug 7. Pflugers Arch. 2017. PMID: 28785802 Free PMC article.
-
Cortical spreading depression impairs oxygen delivery and metabolism in mice.J Cereb Blood Flow Metab. 2012 Feb;32(2):376-86. doi: 10.1038/jcbfm.2011.148. Epub 2011 Oct 19. J Cereb Blood Flow Metab. 2012. PMID: 22008729 Free PMC article.
-
Oxygen and seizure dynamics: II. Computational modeling.J Neurophysiol. 2014 Jul 15;112(2):213-23. doi: 10.1152/jn.00541.2013. Epub 2014 Mar 26. J Neurophysiol. 2014. PMID: 24671540 Free PMC article.
-
Single-cell imaging tools for brain energy metabolism: a review.Neurophotonics. 2014 Jul;1(1):011004. doi: 10.1117/1.NPh.1.1.011004. Epub 2014 May 29. Neurophotonics. 2014. PMID: 26157964 Free PMC article.
-
Glycolysis and oxidative phosphorylation in neurons and astrocytes during network activity in hippocampal slices.J Cereb Blood Flow Metab. 2014 Mar;34(3):397-407. doi: 10.1038/jcbfm.2013.222. Epub 2013 Dec 11. J Cereb Blood Flow Metab. 2014. PMID: 24326389 Free PMC article.
References
-
- Aitken PG, Tombaugh GC, Turner DA, Somjen GG. Similar propagation of SD and hypoxic SD-like depolarization in rat hippocampus recorded optically and electrically. J Neurophysiol. 1998;80:1514–1521. - PubMed
-
- Aubin JE. Autofluorescence of viable cultured mammalian cells. J Histochem Cytochem. 1979;27:36–43. - PubMed
-
- Buerk DG, Nair P. PtiO2 and CMRO2 changes in cortex and hippocampus of aging gerbil brain. J App Physiol. 1993;74:1723–1728. - PubMed
-
- Cerdán S, Rodrigues TB, Sierra A, Benito M, Fonseca LL, Fonseca CP, García-Martín ML. The redox switch/redox coupling hypothesis. Neurochem Int. 2006;48:523–530. - PubMed
-
- Chance B, Cohen P, Jobsis F, Schoener B. Intracellular oxidation-reduction states in vivo. Science. 1962;137:499–508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

