Safety and Feasibility of Carboplatin and Paclitaxel followed by Fluoropyrimidine Analogs and Radiation as Adjuvant Therapy for Gastric Cancer
- PMID: 20737041
- PMCID: PMC2914386
- DOI: 10.1159/000250082
Safety and Feasibility of Carboplatin and Paclitaxel followed by Fluoropyrimidine Analogs and Radiation as Adjuvant Therapy for Gastric Cancer
Abstract
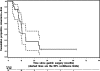
BACKGROUND: Adjuvant 5-fluorouracil (5FU)-based chemo-radiotherapy is currently considered a standard of care for the treatment of gastric cancer. The impact of 5FU-based adjuvant therapy on the rate of distant recurrence has been modest. In order to improve the systemic effects of adjuvant therapy, we have been treating patients with resected gastric cancer with carboplatin and paclitaxel followed by fluoropyrimidine analogue and radiation. METHODS: We report on the outcomes of 21 consecutive gastric cancer patients treated off protocol with adjuvant carboplatin (area under the curve 5 mg/ml x min) and paclitaxel (175-200 mg/m(2)) every 3 weeks, followed by concurrent pyrimidine analogs (either capecitabine 1,600-2,000 mg/m(2)/day in 17 patients, or 5FU 200 mg/m(2)/day in 4 patients) and radiation (45-50.4 Gy). Patients received a total of 4-6 cycles of carboplatin and paclitaxel. RESULTS: The median age at diagnosis was 60 years. Sixteen patients had stage 3 disease and 7 of them had positive surgical margins (6 with R1 and 1 with R2 resection), 3 patients were stage 2, and 2 patients were stage 1 (all had R0 resection). All patients had D1/D2 (4 had D2 and 17 had D1) lymph node dissection. The incidence of grade 3 or higher overall, hematologic, or gastrointestinal toxicity in the patients receiving carboplatin and paclitaxel was 57, 48 and 10%, respectively. No treatment-related deaths were observed. After adjuvant treatment 15 patients developed recurrent disease, 10 of whom had distant metastases. The median recurrence-free survival (RFS) was 12.3 months. The median overall survival (OS) was 16.0 months. Patients with R0 resection had significantly longer OS than did those with positive surgical margins (log-rank p = 0.0060). Median OS for the R0 resection group was 28.8 months. CONCLUSIONS: Carboplatin and paclitaxel added to radiation plus fluoropyrimidine analogs is a well-tolerated regimen in the adjuvant setting. The activity of this regimen in this relatively high-risk group of gastric cancer patients is of interest for future development.
Figures


Similar articles
-
A phase II study of concurrent carboplatin and paclitaxel and thoracic radiotherapy for completely resected stage II and IIIA non-small cell lung cancer.J Thorac Oncol. 2007 Apr;2(4):287-92. doi: 10.1097/01.JTO.0000263710.54073.b3. J Thorac Oncol. 2007. PMID: 17409799 Clinical Trial.
-
Paclitaxel and carboplatin adjuvant therapy alone or with radiotherapy for resected nonsmall cell lung carcinoma: a feasibility study of the Minnie Pearl Cancer Research Network.Cancer. 2001 Oct 15;92(8):2142-7. doi: 10.1002/1097-0142(20011015)92:8<2142::aid-cncr1556>3.0.co;2-r. Cancer. 2001. PMID: 11596031 Clinical Trial.
-
Comparison of neoadjuvant chemoradiation with carboplatin/ paclitaxel or cisplatin/ 5-fluoruracil in patients with squamous cell carcinoma of the esophagus.Radiat Oncol. 2017 Nov 21;12(1):182. doi: 10.1186/s13014-017-0904-y. Radiat Oncol. 2017. PMID: 29157271 Free PMC article.
-
Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: A multicentric phase II study.Int J Radiat Oncol Biol Phys. 2006 Mar 15;64(4):1129-39. doi: 10.1016/j.ijrobp.2005.09.017. Epub 2006 Jan 18. Int J Radiat Oncol Biol Phys. 2006. PMID: 16414206 Clinical Trial.
-
Capecitabine with radiation is an effective adjuvant therapy in gastric cancers.World J Gastroenterol. 2010 Aug 7;16(29):3709-15. doi: 10.3748/wjg.v16.i29.3709. World J Gastroenterol. 2010. PMID: 20677345 Free PMC article.
Cited by
-
Regulatory T-cell density and cytotoxic T lymphocyte density are associated with complete response to neoadjuvant paclitaxel and carboplatin chemoradiotherapy in gastric cancer.Exp Ther Med. 2018 Nov;16(5):3813-3820. doi: 10.3892/etm.2018.6684. Epub 2018 Sep 3. Exp Ther Med. 2018. PMID: 30344657 Free PMC article.
References
-
- Pera M. Epidemiology of esophageal cancer, especially adenocarcinoma of the esophagus and esophagogastric junction. Recent Results Cancer Res. 2000;155:1–14. - PubMed
-
- Kim DY, et al. Predictors of long-term survival in node-positive gastric carcinoma patients with curative resection. Langenbecks Arch Surg. 2007;392:131–134. - PubMed
-
- Maruyama M, et al. Clinicopathological study of gastric carcinoma in high- and low-mortality countries: comparison between Japan and the United States. Gastric Cancer. 1998;1:64–70. - PubMed
-
- Sakaguchi T, et al. Characteristics and clinical outcome of proximal-third gastric cancer. J Am Coll Surg. 1998;187:352–357. - PubMed
-
- Hochwald SN, et al. Analysis of 154 actual five-year survivors of gastric cancer. J Gastrointest Surg. 2000;4:520–525. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

