Spinal injection of TNF-α-activated astrocytes produces persistent pain symptom mechanical allodynia by releasing monocyte chemoattractant protein-1
- PMID: 20737477
- PMCID: PMC2951489
- DOI: 10.1002/glia.21056
Spinal injection of TNF-α-activated astrocytes produces persistent pain symptom mechanical allodynia by releasing monocyte chemoattractant protein-1
Abstract
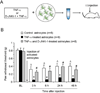
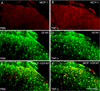
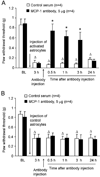
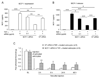
Accumulating evidence suggests that spinal astrocytes play an important role in the genesis of persistent pain, by increasing the activity of spinal cord nociceptive neurons, i.e., central sensitization. However, direct evidence of whether activation of astrocytes is sufficient to induce chronic pain symptoms is lacking. We investigated whether and how spinal injection of activated astrocytes could produce mechanical allodynia, a cardinal feature of chronic pain, in naïve mice. Spinal (intrathecal) injection of astrocytes, which were prepared from cerebral cortexes of neonatal mice and briefly stimulated by tumor necrosis factor-alpha (TNF-α), induced a substantial decrease in paw withdrawal thresholds, indicating the development of mechanical allodynia. This allodynia was prevented when the astrocyte cultures were pretreated with a peptide inhibitor of c-Jun N-terminal kinase (JNK), D-JNKI-1. Of note a short exposure of astrocytes to TNF-α for 15 min dramatically increased the expression and release of the chemokine monocyte chemoattractant protein-1 (MCP-1), even 3 h after TNF-α withdrawal, in a JNK-dependent manner. In parallel, intrathecal administration of TNF-α induced MCP-1 expression in spinal cord astrocytes. In particular, mechanical allodynia induced by TNF-α-activated astrocytes was reversed by a MCP-1 neutralizing antibody. Finally, pretreatment of astrocytes with MCP-1 siRNA attenuated astrocytes-induced mechanical allodynia. Taken together, our results suggest that activated astrocytes are sufficient to produce persistent pain symptom in naïve mice by releasing MCP-1.
© 2010 Wiley-Liss, Inc.
Figures


References
-
- Baron R. Neuropathic pain: a clinical perspective. Handb Exp Pharmacol. 2009:3–30. - PubMed
-
- Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9:1180–1186. - PubMed
-
- Campbell JN, Raja SN, Meyer RA, Mackinnon SE. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988;32:89–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

