Central respiratory chemoreception
- PMID: 20737591
- PMCID: PMC2929977
- DOI: 10.1002/cne.22435
Central respiratory chemoreception
Abstract
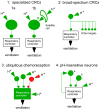
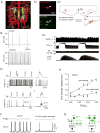
By definition central respiratory chemoreceptors (CRCs) are cells that are sensitive to changes in brain PCO(2) or pH and contribute to the stimulation of breathing elicited by hypercapnia or metabolic acidosis. CO(2) most likely works by lowering pH. The pertinent proton receptors have not been identified and may be ion channels. CRCs are probably neurons but may also include acid-sensitive glia and vascular cells that communicate with neurons via paracrine mechanisms. Retrotrapezoid nucleus (RTN) neurons are the most completely characterized CRCs. Their high sensitivity to CO(2) in vivo presumably relies on their intrinsic acid sensitivity, excitatory inputs from the carotid bodies and brain regions such as raphe and hypothalamus, and facilitating influences from neighboring astrocytes. RTN neurons are necessary for the respiratory network to respond to CO(2) during the perinatal period and under anesthesia. In conscious adults, RTN neurons contribute to an unknown degree to the pH-dependent regulation of breathing rate, inspiratory, and expiratory activity. The abnormal prenatal development of RTN neurons probably contributes to the congenital central hypoventilation syndrome. Other CRCs presumably exist, but the supportive evidence is less complete. The proposed locations of these CRCs are the medullary raphe, the nucleus tractus solitarius, the ventrolateral medulla, the fastigial nucleus, and the hypothalamus. Several wake-promoting systems (serotonergic and catecholaminergic neurons, orexinergic neurons) are also putative CRCs. Their contribution to central respiratory chemoreception may be behavior dependent or vary according to the state of vigilance.
Figures

References
-
- Ahmad HR, Loeschcke HH. Transient and steady state responses of pulmonary ventilation to the medullary extracellular pH after approximately rectangular changes in alveolar PCO2. Pflugers Arch. 1982;395:285–292. - PubMed
-
- Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1473–R1495. - PubMed
-
- Aller MI, Wisden W. Changes in expression of some two-pore domain potassium channel genes (KCNK) in selected brain regions of developing mice. Neurosci. 2008;151:1154–1172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

