Neuronal DNA content variation (DCV) with regional and individual differences in the human brain
- PMID: 20737596
- PMCID: PMC2932632
- DOI: 10.1002/cne.22436
Neuronal DNA content variation (DCV) with regional and individual differences in the human brain
Abstract
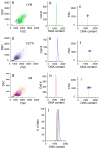
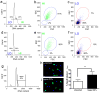
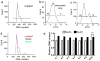
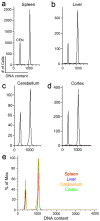
It is widely assumed that the human brain contains genetically identical cells through which postgenomic mechanisms contribute to its enormous diversity and complexity. The relatively recent identification of neural cells throughout the neuraxis showing somatically generated mosaic aneuploidy indicates that the vertebrate brain can be genomically heterogeneous (Rehen et al. [2001] Proc. Natl. Acad. Sci. U. S. A. 98:13361-13366; Rehen et al. [2005] J. Neurosci. 25:2176-2180; Yurov et al. [2007] PLoS ONE:e558; Westra et al. [2008] J. Comp. Neurol. 507:1944-1951). The extent of human neural aneuploidy is currently unknown because of technically limited sample sizes, but is reported to be small (Iourov et al. [2006] Int. Rev. Cytol. 249:143-191). During efforts to interrogate larger cell populations by using DNA content analyses, a surprising result was obtained: human frontal cortex brain cells were found to display "DNA content variation (DCV)" characterized by an increased range of DNA content both in cell populations and within single cells. On average, DNA content increased by approximately 250 megabases, often representing a substantial fraction of cells within a given sample. DCV within individual human brains showed regional variation, with increased prevalence in the frontal cortex and less variation in the cerebellum. Further, DCV varied between individual brains. These results identify DCV as a new feature of the human brain, encompassing and further extending genomic alterations produced by aneuploidy, which may contribute to neural diversity in normal and pathophysiological states, altered functions of normal and disease-linked genes, and differences among individuals.
Figures







Similar articles
-
The genomically mosaic brain: aneuploidy and more in neural diversity and disease.Semin Cell Dev Biol. 2013 Apr;24(4):357-69. doi: 10.1016/j.semcdb.2013.02.003. Epub 2013 Mar 4. Semin Cell Dev Biol. 2013. PMID: 23466288 Free PMC article. Review.
-
Regional mosaic genomic heterogeneity in the elderly and in Alzheimer's disease as a correlate of neuronal vulnerability.Acta Neuropathol. 2015 Oct;130(4):501-10. doi: 10.1007/s00401-015-1465-5. Epub 2015 Aug 23. Acta Neuropathol. 2015. PMID: 26298468
-
Changes in neuronal DNA content variation in the human brain during aging.Aging Cell. 2012 Aug;11(4):628-33. doi: 10.1111/j.1474-9726.2012.00826.x. Epub 2012 May 24. Aging Cell. 2012. PMID: 22510449
-
Constitutional aneuploidy in the normal human brain.J Neurosci. 2005 Mar 2;25(9):2176-80. doi: 10.1523/JNEUROSCI.4560-04.2005. J Neurosci. 2005. PMID: 15745943 Free PMC article.
-
Systems biology of aging.Adv Exp Med Biol. 2015;847:163-78. doi: 10.1007/978-1-4939-2404-2_8. Adv Exp Med Biol. 2015. PMID: 25916591 Review.
Cited by
-
Genomic mosaicism with increased amyloid precursor protein (APP) gene copy number in single neurons from sporadic Alzheimer's disease brains.Elife. 2015 Feb 4;4:e05116. doi: 10.7554/eLife.05116. Elife. 2015. PMID: 25650802 Free PMC article.
-
A direct comparison of interphase FISH versus low-coverage single cell sequencing to detect aneuploidy reveals respective strengths and weaknesses.Sci Rep. 2019 Jul 19;9(1):10508. doi: 10.1038/s41598-019-46606-w. Sci Rep. 2019. PMID: 31324840 Free PMC article.
-
Epigenetic and Cellular Diversity in the Brain through Allele-Specific Effects.Trends Neurosci. 2018 Dec;41(12):925-937. doi: 10.1016/j.tins.2018.07.005. Epub 2018 Aug 8. Trends Neurosci. 2018. PMID: 30098802 Free PMC article. Review.
-
Mosaic APP Gene Recombination in Alzheimer's Disease-What's Next?J Exp Neurosci. 2019 May 16;13:1179069519849669. doi: 10.1177/1179069519849669. eCollection 2019. J Exp Neurosci. 2019. PMID: 31205422 Free PMC article.
-
Genomic Indexing by Somatic Gene Recombination of mRNA/ncRNA - Does It Play a Role in Genomic Mosaicism, Memory Formation, and Alzheimer's Disease?Front Genet. 2020 Apr 29;11:370. doi: 10.3389/fgene.2020.00370. eCollection 2020. Front Genet. 2020. PMID: 32411177 Free PMC article. Review.
References
-
- Aldhous MC, Raab GM, Doherty KV, Mok JY, Bird AG, Froebel KS. Age-related ranges of memory, activation, and cytotoxic markers on CD4 and CD8 cells in children. Journal of clinical immunology. 1994;14(5):289–298. - PubMed
-
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Bjork-Eriksson T, Nordborg C, Gage FH, Druid H, Eriksson PS, Frisen J. Neocortical neurogenesis in humans is restricted to development. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(33):12564–12568. - PMC - PubMed
-
- Brasseur R, de Kruijff B, Ruysschaert JM. Mode of organization of lipid aggregates: a conformational analysis. Biosci Rep. 1984;4(3):259–267. - PubMed
-
- Bruner JM, Langford LA, Fuller GN. Neuropathology, cell biology, and newer diagnostic methods. Current opinion in oncology. 1993;5(3):441–449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

