Biological evaluation of pH-responsive polymer-caged nanobins for breast cancer therapy
- PMID: 20738118
- PMCID: PMC3650137
- DOI: 10.1021/nn100560p
Biological evaluation of pH-responsive polymer-caged nanobins for breast cancer therapy
Abstract
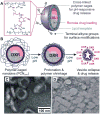
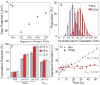
A series of doxorubicin-loaded polymer-caged nanobins (PCN(DXR)) were evaluated in vivo in a murine MDA-MB-231 xenograft model of triple-negative breast cancer. The cross-linked polymer cage in PCN(DXR) offers protection for the drug payload while serving as a pH-responsive trigger that enhances drug release in the acidic environments commonly seen in solid tumors and endosomes. Varying the degree of cross-linking in the polymer cage allows the surface potential of PCN(DXR), and thus the in vivo circulation lifetime of the nanocarriers, to be tuned in a facile fashion. Given these design advantages, the present study provides the first in vivo evidence that PCN(DXR) can effectively inhibit tumor growth in a murine model of breast cancer. Importantly, PCN(DXR) was well-tolerated by mice, and drug encapsulation attenuated the toxicity of free doxorubicin. Taken together, this study demonstrates the potential utility of the PCN platform in cancer therapy.
Figures


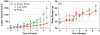

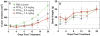
Comment in
-
Nanoparticulate alternatives for drug delivery.ACS Nano. 2010 Sep 28;4(9):4967-70. doi: 10.1021/nn102324e. ACS Nano. 2010. PMID: 20873786 Review.
Similar articles
-
Triggered release of pharmacophores from [Ni(HAsO₃)]-loaded polymer-caged nanobin enhances pro-apoptotic activity: a combined experimental and theoretical study.ACS Nano. 2011 May 24;5(5):3961-9. doi: 10.1021/nn200478m. Epub 2011 May 9. ACS Nano. 2011. PMID: 21466214 Free PMC article.
-
Polymer-caged nanobins for synergistic cisplatin-doxorubicin combination chemotherapy.J Am Chem Soc. 2010 Dec 8;132(48):17130-8. doi: 10.1021/ja107333g. Epub 2010 Nov 15. J Am Chem Soc. 2010. PMID: 21077673 Free PMC article.
-
"Clickable" polymer-caged nanobins as a modular drug delivery platform.J Am Chem Soc. 2009 Jul 8;131(26):9311-20. doi: 10.1021/ja9017336. J Am Chem Soc. 2009. PMID: 19527027 Free PMC article.
-
Bone-targeted doxorubicin-loaded nanoparticles as a tool for the treatment of skeletal metastases.Curr Cancer Drug Targets. 2010 Nov;10(7):649-59. doi: 10.2174/156800910793605767. Curr Cancer Drug Targets. 2010. PMID: 20578992
-
Using Peptide Aptamer Targeted Polymers as a Model Nanomedicine for Investigating Drug Distribution in Cancer Nanotheranostics.Mol Pharm. 2017 Oct 2;14(10):3539-3549. doi: 10.1021/acs.molpharmaceut.7b00560. Epub 2017 Sep 7. Mol Pharm. 2017. PMID: 28880092
Cited by
-
Targeted nanoparticles for image-guided treatment of triple-negative breast cancer: clinical significance and technological advances.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015 Nov-Dec;7(6):797-816. doi: 10.1002/wnan.1343. Epub 2015 May 12. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015. PMID: 25966677 Free PMC article. Review.
-
pH-Responsive Theranostic Polymer-Caged Nanobins (PCNs): Enhanced Cytotoxicity and T1 MRI Contrast by Her2-Targeting.Part Part Syst Charact. 2013 Sep;30(9):770-774. doi: 10.1002/ppsc.201300158. Part Part Syst Charact. 2013. PMID: 24516291 Free PMC article.
-
Smart Nanoscale Drug Delivery Platforms from Stimuli-Responsive Polymers and Liposomes.Macromolecules. 2013 Dec 10;46(23):9169-9180. doi: 10.1021/ma401529w. Epub 2013 Nov 27. Macromolecules. 2013. PMID: 28804160 Free PMC article.
-
Triggered release of pharmacophores from [Ni(HAsO₃)]-loaded polymer-caged nanobin enhances pro-apoptotic activity: a combined experimental and theoretical study.ACS Nano. 2011 May 24;5(5):3961-9. doi: 10.1021/nn200478m. Epub 2011 May 9. ACS Nano. 2011. PMID: 21466214 Free PMC article.
-
Recent advances in nanotheranostics for triple negative breast cancer treatment.J Exp Clin Cancer Res. 2019 Oct 28;38(1):430. doi: 10.1186/s13046-019-1443-1. J Exp Clin Cancer Res. 2019. PMID: 31661003 Free PMC article. Review.
References
-
- Allen TM, Cullis PR. Drug Delivery Systems: Entering the Mainstream. Science. 2004;303:1818–1822. - PubMed
-
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat Nanotechnol. 2007;2:751–760. - PubMed
-
- Davis ME, Chen Z, Shin DM. Nanoparticle Therapeutics: An Emerging Treatment Modality for Cancer. Nat Rev Drug Discov. 2008;7:771–782. - PubMed
-
- Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing Liposomes for Delivery of Chemotherapeutic Agents to Solid Tumors. Pharmacol Rev. 1999;51:691–744. - PubMed
-
- Waterhouse DN, Tardi PG, Mayer LD, Bally MB. A Comparison of Liposomal Formulations of Doxorubicin with Drug Administered in Free Form: Changing Toxicity Profiles. Drug Saf. 2001;24:903–920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

