Inhibition of the mitochondrial permeability transition by protein kinase A in rat liver mitochondria and hepatocytes
- PMID: 20738255
- PMCID: PMC3501339
- DOI: 10.1042/BJ20091741
Inhibition of the mitochondrial permeability transition by protein kinase A in rat liver mitochondria and hepatocytes
Abstract
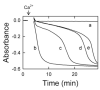
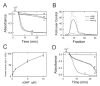
NO and cGMP administered at reperfusion after ischaemia prevent injury to hepatocytes mediated by the MPT (mitochondrial permeability transition). To characterize further the mechanism of protection, the ability of hepatic cytosol in combination with cyclic nucleotides to delay onset of the calcium-induced MPT was evaluated in isolated rat liver mitochondria. Liver cytosol plus cGMP or cAMP dose-dependently inhibited the MPT, required ATP hydrolysis for inhibition and did not inhibit mitochondrial calcium uptake. Specific peptide inhibitors for PKA (protein kinase A), but not PKG (protein kinase G), abolished cytosol-induced inhibition of MPT onset. Activity assays showed a cGMP- and cAMP-stimulated protein kinase activity in liver cytosol that was completely inhibited by PKI, a PKA peptide inhibitor. Size-exclusion chromatography of liver cytosol produced a single peak of cGMP/cAMP-stimulated kinase activity with an estimated protein size of 180-220 kDa. This fraction was PKI-sensitive and delayed onset of the MPT. Incubation of active catalytic PKA subunit directly with mitochondria in the absence of cytosol and cyclic nucleotide also delayed MPT onset, and incubation with purified outer membranes led to phosphorylation of a major 31 kDa band. After ischaemia, administration at reperfusion of membrane-permeant cAMPs and cAMP-mobilizing glucagon prevented reperfusion injury to hepatocytes. In conclusion, PKA in liver cytosol activated by cGMP or cAMP acts directly on mitochondria to delay onset of the MPT and protect hepatocytes from cell death after ischaemia/reperfusion.
Figures






Similar articles
-
Nitric oxide protects rat hepatocytes against reperfusion injury mediated by the mitochondrial permeability transition.Hepatology. 2004 Jun;39(6):1533-43. doi: 10.1002/hep.20197. Hepatology. 2004. PMID: 15185294
-
Mitochondrial permeability transition in the switch from necrotic to apoptotic cell death in ischemic rat hepatocytes.Gastroenterology. 2003 Feb;124(2):494-503. doi: 10.1053/gast.2003.50059. Gastroenterology. 2003. PMID: 12557154
-
Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes.Am J Physiol. 1997 Dec;273(6):C1783-92. doi: 10.1152/ajpcell.1997.273.6.C1783. Am J Physiol. 1997. PMID: 9435481
-
Role of the mitochondrial permeability transition in apoptotic and necrotic death after ischemia/reperfusion injury to hepatocytes.Curr Mol Med. 2003 Sep;3(6):527-35. doi: 10.2174/1566524033479564. Curr Mol Med. 2003. PMID: 14527084 Review.
-
The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy.Biochim Biophys Acta. 1998 Aug 10;1366(1-2):177-96. doi: 10.1016/s0005-2728(98)00112-1. Biochim Biophys Acta. 1998. PMID: 9714796 Review.
Cited by
-
Molecular mechanisms and consequences of mitochondrial permeability transition.Nat Rev Mol Cell Biol. 2022 Apr;23(4):266-285. doi: 10.1038/s41580-021-00433-y. Epub 2021 Dec 8. Nat Rev Mol Cell Biol. 2022. PMID: 34880425 Review.
-
Phosphorylation of voltage-dependent anion channel by serine/threonine kinases governs its interaction with tubulin.PLoS One. 2011;6(10):e25539. doi: 10.1371/journal.pone.0025539. Epub 2011 Oct 13. PLoS One. 2011. PMID: 22022409 Free PMC article.
-
Tissue-specific differences impacts therapeutic outcomes of mitochondrial transplantation through regulation of bioenergetics in metabolic syndrome.Cell Tissue Res. 2025 Aug;401(2):213-228. doi: 10.1007/s00441-025-03977-z. Epub 2025 May 26. Cell Tissue Res. 2025. PMID: 40415076
-
Rewarming Injury after Cold Preservation.Int J Mol Sci. 2019 Apr 26;20(9):2059. doi: 10.3390/ijms20092059. Int J Mol Sci. 2019. PMID: 31027332 Free PMC article. Review.
-
Pre-conditions for eliminating mitochondrial dysfunction and maintaining liver function after hepatic ischaemia reperfusion.J Cell Mol Med. 2017 Sep;21(9):1719-1731. doi: 10.1111/jcmm.13129. Epub 2017 Mar 16. J Cell Mol Med. 2017. PMID: 28301072 Free PMC article. Review.
References
-
- Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am. J. Physiol Gastrointest. Liver Physiol. 2003;284:G15–G26. - PubMed
-
- Kim JS, He L, Qian T, Lemasters JJ. Role of the mitochondrial permeability transition in apoptotic and necrotic death after ischemia/reperfusion injury to hepatocytes. Curr. Mol. Med. 2003;3:527–535. - PubMed
-
- Suleiman MS, Halestrap AP, Griffiths EJ. Mitochondria: a target for myocardial protection. Pharmacol. Ther. 2001;89:29–46. - PubMed
-
- Theruvath TP, Zhong Z, Pediaditakis P, Ramshesh VK, Currin RT, Tikunov A, Holmuhamedov E, Lemasters JJ. Minocycline and N-methyl-4-isoleucine cyclosporin (NIM811) mitigate storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology. 2009;47:236–246. - PMC - PubMed
-
- Qian T, Nieminen AL, Herman B, Lemasters JJ. Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes. Am. J. Physiol. 1997;273:C1783–C1792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 DK071477/DK/NIDDK NIH HHS/United States
- DK37034/DK/NIDDK NIH HHS/United States
- R01 DK079879/DK/NIDDK NIH HHS/United States
- P30 DK034987/DK/NIDDK NIH HHS/United States
- R56 DK037034/DK/NIDDK NIH HHS/United States
- DK071477/DK/NIDDK NIH HHS/United States
- R01 DK070844/DK/NIDDK NIH HHS/United States
- DK073336/DK/NIDDK NIH HHS/United States
- R37 DK037034/DK/NIDDK NIH HHS/United States
- DK34987/DK/NIDDK NIH HHS/United States
- DK070844/DK/NIDDK NIH HHS/United States
- R01 DK037034/DK/NIDDK NIH HHS/United States
- R01 DK073336/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources

