Human cytomegalovirus-specific CD8(+) T-cell expansions contain long-lived cells that retain functional capacity in both young and elderly subjects
- PMID: 20738423
- PMCID: PMC3015072
- DOI: 10.1111/j.1365-2567.2010.03334.x
Human cytomegalovirus-specific CD8(+) T-cell expansions contain long-lived cells that retain functional capacity in both young and elderly subjects
Abstract
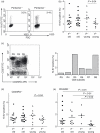
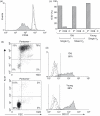
The immune response to human cytomegalovirus (HCMV) infection is characterized by the accumulation of HCMV-specific CD8(+) T cells, particularly in the elderly; such expansions may impair immune responses to other pathogens. We investigated mechanisms underlying HCMV-specific expansions in 12 young and 21 old healthy subjects (although not all analyses were performed on all subjects). Phenotypically, HCMV-pentamer(+) CD8(+) T cells were characterized by marked Vβ restriction, advanced differentiation (being predominantly CD27(-) CD28(-) ), and variable CD45RO/RA expression. Although more common and larger in older subjects, expansions had similar phenotypic characteristics in the young. In one old subject, repeated studies demonstrated stability in size and Vβ distribution of pentamer(+) populations over 6 years. We tested whether HCMV-specific CD8(+) T-cell expansions arose from accelerated proliferation or extended lifespan by in vivo labelling with deuterated glucose and ex vivo Ki-67 expression. Uptake of deuterated glucose was lower in pentamer(+) cells than in pentamer(-) CD8(+) CD45RO(+) or CD8(+) CD45RA(+) cells in three old subjects, consistent with reduced proliferation and extended lifespan. Similarly Ki-67 labelling showed no evidence for increased proliferation in HCMV-specific CD8(+) expansions in older subjects, although pentamer(-) CD45RA(+) cells from young donors expressed very little Ki-67. We investigated Bcl-2 and CD95 as possible anti-apoptotic mediators, but neither was associated with pentamer-positivity. To investigate whether expansion represents a compensatory response to impaired functionality, we performed two tests of functionality, peptide-stimulated proliferation and CD107 expression; both were intact in pentamer(+) cells. Our data suggest that HCMV-specific CD8(+) expansions in older subjects accumulate by extended lifespan, rather than accelerated proliferation.
Figures




References
-
- Sinclair J, Sissons P. Latency and reactivation of human cytomegalovirus. J Gen Virol. 2006;87:1763–79. - PubMed
-
- Waller EC, Day E, Sissons JG, Wills MR. Dynamics of T cell memory in human cytomegalovirus infection. Med Microbiol Immunol. 2008;197:83–96. - PubMed
-
- Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat Immunol. 2005;6:873–9. - PubMed
-
- Pawelec G, Larbi A, Derhovanessian E. Senescence of the human immune system. J Comp Pathol. 2010;142S:S39–44. - PubMed
-
- Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev. 2005;205:257–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

