Functional and cellular characterization of human Retinoic Acid Induced 1 (RAI1) mutations associated with Smith-Magenis Syndrome
- PMID: 20738874
- PMCID: PMC2939504
- DOI: 10.1186/1471-2199-11-63
Functional and cellular characterization of human Retinoic Acid Induced 1 (RAI1) mutations associated with Smith-Magenis Syndrome
Abstract
Background: Smith-Magenis Syndrome is a contiguous gene syndrome in which the dosage sensitive gene has been identified: the Retinoic Acid Induced 1 (RAI1). Little is known about the function of human RAI1.
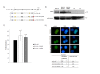
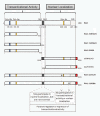
Results: We generated the full-length cDNA of the wild type protein and five mutated forms: RAI1-HA 2687delC, RAI1-HA 3103delC, RAI1 R960X, RAI1-HA Q1562R, and RAI1-HA S1808N. Four of them have been previously associated with SMS clinical phenotype. Molecular weight, subcellular localization and transcription factor activity of the wild type and mutant forms were studied by western blot, immunofluorescence and luciferase assays respectively. The wild type protein and the two missense mutations presented a higher molecular weight than expected, localized to the nucleus and activated transcription of a reporter gene. The frameshift mutations generated a truncated polypeptide with transcription factor activity but abnormal subcellular localization, and the same was true for the 1-960aa N-terminal half of RAI1. Two different C-terminal halves of the RAI1 protein (1038aa-end and 1229aa-end) were able to localize into the nucleus but had no transactivation activity.
Conclusion: Our results indicate that transcription factor activity and subcellular localization signals reside in two separate domains of the protein and both are essential for the correct functionality of RAI1. The pathogenic outcome of some of the mutated forms can be explained by the dissociation of these two domains.
Figures



References
-
- Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, Andrews TD, Fiegler H, Shapero MH, Carson AR, Chen W, Cho EK, Dallaire S, Freeman JL, González JR, Gratacòs M, Huang J, Kalaitzopoulos D, Komura D, MacDonald JR, Marshall CR, Mei R, Montgomery L, Nishimura K, Okamura K, Shen F, Somerville MJ, Tchinda J, Valsesia A, Woodwark C, Yang F. et al. Global variation in copy number in the human genome. Nature. 2006;444:444. doi: 10.1038/nature05329. - DOI - PMC - PubMed
-
- Korn JM, Kuruvilla FG, McCarroll SA, Wysoker A, Nemesh J, Cawley S, Hubbell E, Veitch J, Collins PJ, Darvishi K, Lee C, Nizzari MM, Gabriel SB, Purcell S, Daly MJ, Altshuler D. Integrated genotype calling and association analysis of SNPs, common copy number polymorphisms and rare CNVs. Nat Genet. 2008;40:1253. doi: 10.1038/ng.237. - DOI - PMC - PubMed
-
- McCarroll SA, Kuruvilla FG, Korn JM, Cawley S, Nemesh J, Wysoker A, Shapero MH, de Bakker PI, Maller JB, Kirby A, Elliott AL, Parkin M, Hubbell E, Webster T, Mei R, Veitch J, Collins PJ, Handsaker R, Lincoln S, Nizzari M, Blume J, Jones KW, Rava R, Daly MJ, Gabriel SB, Altshuler D. Integrated detection and population-genetic analysis of SNPs and copy number variation. Nat Genet. 2008;40:1166. doi: 10.1038/ng.238. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

