Epigenetic silencing of beta-spectrin, a TGF-beta signaling/scaffolding protein in a human cancer stem cell disorder: Beckwith-Wiedemann syndrome
- PMID: 20739274
- PMCID: PMC2975233
- DOI: 10.1074/jbc.M110.162347
Epigenetic silencing of beta-spectrin, a TGF-beta signaling/scaffolding protein in a human cancer stem cell disorder: Beckwith-Wiedemann syndrome
Abstract
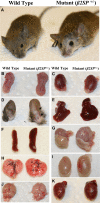
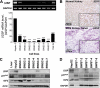
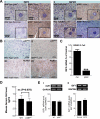
Hereditary cancer syndromes provide powerful insights into dysfunctional signaling pathways that lead to sporadic cancers. Beckwith-Wiedemann syndrome (BWS) is a hereditary human cancer stem cell syndrome currently linked to deregulated imprinting at chromosome 11p15 and uniparental disomy. However, causal molecular defects and genetic models have remained elusive to date in the majority of cases. The non-pleckstrin homology domain β-spectrin (β2SP) (the official name for human is Spectrin, beta, nonerythrocytic 1 (SPTBN1), isoform 2; the official name for mouse is Spectrin beta 2 (Spnb2), isoform 2), a scaffolding protein, functions as a potent TGF-β signaling member adaptor in tumor suppression and development. Yet, the role of the β2SP in human tumor syndromes remains unclear. Here, we report that β2SP(+/-) mice are born with many phenotypic characteristics observed in BWS patients, suggesting that β2SP mutant mice phenocopy BWS, and β2SP loss could be one of the mechanisms associated with BWS. Our results also suggest that epigenetic silencing of β2SP is a new potential causal factor in human BWS patients. Furthermore, β2SP(+/-) mice provide an important animal model for BWS, as well as sporadic cancers associated with it, including lethal gastrointestinal and pancreatic cancer. Thus, these studies could lead to further insight into defects generated by dysfunctional stem cells and identification of new treatment strategies and functional markers for the early detection of these lethal cancers that otherwise cannot be detected at an early stage.
Figures


Similar articles
-
TGF-β/β2-spectrin/CTCF-regulated tumor suppression in human stem cell disorder Beckwith-Wiedemann syndrome.J Clin Invest. 2016 Feb;126(2):527-42. doi: 10.1172/JCI80937. Epub 2016 Jan 19. J Clin Invest. 2016. PMID: 26784546 Free PMC article.
-
Epigenotype-phenotype correlations in Beckwith-Wiedemann syndrome.J Med Genet. 2000 Dec;37(12):921-6. doi: 10.1136/jmg.37.12.921. J Med Genet. 2000. PMID: 11106355 Free PMC article.
-
Beckwith-Wiedemann syndrome and uniparental disomy 11p: fine mapping of the recombination breakpoints and evaluation of several techniques.Eur J Hum Genet. 2011 Apr;19(4):416-21. doi: 10.1038/ejhg.2010.236. Epub 2011 Jan 19. Eur J Hum Genet. 2011. PMID: 21248736 Free PMC article.
-
Clinical and Molecular Diagnosis of Beckwith-Wiedemann Syndrome with Single- or Multi-Locus Imprinting Disturbance.Int J Mol Sci. 2021 Mar 26;22(7):3445. doi: 10.3390/ijms22073445. Int J Mol Sci. 2021. PMID: 33810554 Free PMC article. Review.
-
Beckwith-Wiedemann syndrome.Am J Med Genet C Semin Med Genet. 2010 Aug 15;154C(3):343-54. doi: 10.1002/ajmg.c.30267. Am J Med Genet C Semin Med Genet. 2010. PMID: 20803657 Review.
Cited by
-
Transformation-induced stress at telomeres is counteracted through changes in the telomeric proteome including SAMHD1.Life Sci Alliance. 2018 Jul 17;1(4):e201800121. doi: 10.26508/lsa.201800121. eCollection 2018 Aug. Life Sci Alliance. 2018. PMID: 30456372 Free PMC article.
-
Analysis of Genomes and Transcriptomes of Hepatocellular Carcinomas Identifies Mutations and Gene Expression Changes in the Transforming Growth Factor-β Pathway.Gastroenterology. 2018 Jan;154(1):195-210. doi: 10.1053/j.gastro.2017.09.007. Epub 2017 Sep 15. Gastroenterology. 2018. PMID: 28918914 Free PMC article.
-
Dysregulated PJA1-TGF-β signaling in cancer stem cell-associated liver cancers.Oncoscience. 2020 Nov 30;7(11-12):88-95. doi: 10.18632/oncoscience.522. eCollection 2020 Nov. Oncoscience. 2020. PMID: 33457451 Free PMC article.
-
Preclinical and Clinical Epigenetic-Based Reconsideration of Beckwith-Wiedemann Syndrome.Front Genet. 2020 Sep 15;11:563718. doi: 10.3389/fgene.2020.563718. eCollection 2020. Front Genet. 2020. PMID: 33101381 Free PMC article. Review.
-
Transforming growth factor-β in liver cancer stem cells and regeneration.Hepatol Commun. 2017 Jul 21;1(6):477-493. doi: 10.1002/hep4.1062. eCollection 2017 Aug. Hepatol Commun. 2017. PMID: 29404474 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

