Progesterone treatment inhibits and dihydrotestosterone (DHT) treatment potentiates voltage-gated calcium currents in gonadotropin-releasing hormone (GnRH) neurons
- PMID: 20739401
- PMCID: PMC2954728
- DOI: 10.1210/en.2010-0385
Progesterone treatment inhibits and dihydrotestosterone (DHT) treatment potentiates voltage-gated calcium currents in gonadotropin-releasing hormone (GnRH) neurons
Abstract
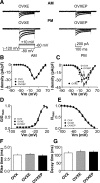
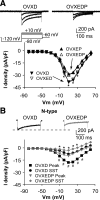
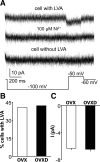
GnRH neurons are central regulators of fertility, and their activity is modulated by steroid feedback. In normal females, GnRH secretion is regulated by estradiol and progesterone (P). Excess androgens present in hyperandrogenemic fertility disorders may disrupt communication of negative feedback signals from P and/or independently stimulate GnRH release. Voltage-gated calcium channels (VGCCs) are important in regulating excitability and hormone release. Estradiol alters VGCCs in a time-of-day-dependent manner. To further elucidate ovarian steroid modulation of GnRH neuron VGCCs, we studied the effects of dihydrotestosterone (DHT) and P. Adult mice were ovariectomized (OVX) or OVX and treated with implants containing DHT (OVXD), estradiol (OVXE), estradiol and DHT (OVXED), estradiol and P (OVXEP), or estradiol, DHT, and P (OVXEDP). Macroscopic calcium current (I(Ca)) was recorded in the morning or afternoon 8-12 d after surgery using whole-cell voltage-clamp. I(Ca) was increased in afternoon vs. morning in GnRH neurons from OVXE mice but this increase was abolished in cells from OVXEP mice. I(Ca) in cells from OVXD mice was increased regardless of time of day; there was no additional effect in OVXED mice. P reduced N-type and DHT potentiated N- and R-type VGCCs; P blocked the DHT potentiation of N-type-mediated current. These data suggest P and DHT have opposing actions on VGCCs in GnRH neurons, but in the presence of both steroids, P dominates. VGCCs are targets of ovarian steroid feedback modulation of GnRH neuron activity and, more specifically, a potential mechanism whereby androgens could activate GnRH neuronal function.
Figures



Similar articles
-
GABAergic integration of progesterone and androgen feedback to gonadotropin-releasing hormone neurons.Biol Reprod. 2005 Jan;72(1):33-41. doi: 10.1095/biolreprod.104.033126. Epub 2004 Sep 1. Biol Reprod. 2005. PMID: 15342358
-
Diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons.J Neurosci. 2010 Mar 17;30(11):3912-23. doi: 10.1523/JNEUROSCI.6256-09.2010. J Neurosci. 2010. PMID: 20237262 Free PMC article.
-
Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback.Endocrinology. 2006 Mar;147(3):1474-9. doi: 10.1210/en.2005-1029. Epub 2005 Dec 8. Endocrinology. 2006. PMID: 16339200
-
Steroid regulation of GnRH neurons.Ann N Y Acad Sci. 2003 Dec;1007:143-52. doi: 10.1196/annals.1286.014. Ann N Y Acad Sci. 2003. PMID: 14993048 Review.
-
Regulation of endogenous conductances in GnRH neurons by estrogens.Brain Res. 2010 Dec 10;1364:25-34. doi: 10.1016/j.brainres.2010.08.096. Epub 2010 Sep 25. Brain Res. 2010. PMID: 20816765 Free PMC article. Review.
Cited by
-
Two types of burst firing in gonadotrophin-releasing hormone neurones.J Neuroendocrinol. 2012 Jul;24(7):1065-77. doi: 10.1111/j.1365-2826.2012.02313.x. J Neuroendocrinol. 2012. PMID: 22435872 Free PMC article.
-
Estradiol Increases Glutamate and GABA Neurotransmission into GnRH Neurons via Retrograde NO-Signaling in Proestrous Mice during the Positive Estradiol Feedback Period.eNeuro. 2018 Aug 3;5(4):ENEURO.0057-18.2018. doi: 10.1523/ENEURO.0057-18.2018. eCollection 2018 Jul-Aug. eNeuro. 2018. PMID: 30079374 Free PMC article.
-
Hypothalamic transcriptional expression of the kisspeptin system and sex steroid receptors differs among polycystic ovary syndrome rat models with different endocrine phenotypes.Clinics (Sao Paulo). 2017 Aug;72(8):510-514. doi: 10.6061/clinics/2017(08)09. Clinics (Sao Paulo). 2017. PMID: 28954011 Free PMC article.
-
Identified GnRH neuron electrophysiology: a decade of study.Brain Res. 2010 Dec 10;1364:10-24. doi: 10.1016/j.brainres.2010.09.066. Epub 2010 Nov 1. Brain Res. 2010. PMID: 20920482 Free PMC article. Review.
-
Reproductive neuroendocrine dysfunction in polycystic ovary syndrome: insight from animal models.Front Neuroendocrinol. 2014 Oct;35(4):494-511. doi: 10.1016/j.yfrne.2014.04.002. Epub 2014 Apr 18. Front Neuroendocrinol. 2014. PMID: 24747343 Free PMC article. Review.
References
-
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E 1978 Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science 202:631–633 - PubMed
-
- Moenter SM, Caraty A, Locatelli A, Karsch FJ 1991 Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology 129:1175–1182 - PubMed
-
- Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E 1981 Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109:376–385 - PubMed
-
- Marshall JC, Griffin ML 1993 The role of changing pulse frequency in the regulation of ovulation. Hum Reprod 8:57–61 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

