The thyroid hormone receptor alpha1 protein is expressed in embryonic postmitotic neurons and persists in most adult neurons
- PMID: 20739404
- PMCID: PMC5417394
- DOI: 10.1210/me.2010-0175
The thyroid hormone receptor alpha1 protein is expressed in embryonic postmitotic neurons and persists in most adult neurons
Abstract
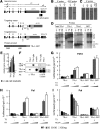
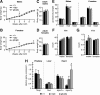
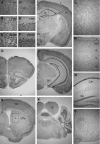
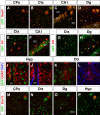
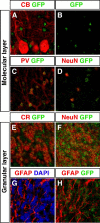
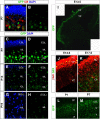
Thyroid hormone is essential for brain development where it acts mainly through the thyroid hormone receptor α1 (TRα1) isoform. However, the potential for the hormone to act in adult neurons has remained undefined due to difficulties in reliably determining the expression pattern of TR proteins in vivo. We therefore created a mouse strain that expresses TRα1 and green fluorescent protein as a chimeric protein from the Thra locus, allowing examination of TRα1 expression during fetal and postnatal development and in the adult. Furthermore, the use of antibodies against other markers enabled identification of TRα1 expression in subtypes of neurons and during specific stages of their maturation. TRα1 expression was first detected in postmitotic cells of the cortical plate in the embryonic telencephalon and preceded the expression of the mature neuronal protein NeuN. In the cerebellum, TRα1 expression was absent in proliferating cells of the external granular layer, but switched on as the cells migrated towards the internal granular layer. In addition, TRα1 was expressed transiently in developing Purkinje cells, but not in mature cells. Glial expression was found in tanycytes in the hypothalamus and in the cerebellum. In the adult brain, TRα1 expression was detected in essentially all neurons. Our data demonstrate that thyroid hormone, unexpectedly, has the capacity to play an important role in virtually all developing and adult neurons. Because the role of TRα1 in most neuronal cell types in vivo is largely unknown, our findings suggest that novel functions for thyroid hormone remain to be identified in the brain.
Figures
References
-
- Delange FM1996. Endemic cretinism. In: Braverman LE, Utiger RD eds. Werner and Ingbar’s the thyroid, 7th ed. Philadelphia: Lippincott-Raven Publishers; 756–767
-
- DeLong GR1996. The neuromuscular system and brain in hypothyroidism. In: Braverman LE, Utiger RD, eds. Werner and Ingbar’s the thyroid, 7th ed. Philadelphia: Lippincott-Raven Publishers; 826–835
-
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F2004. Role of thyroid hormone during early brain development. Eur J Endocrinol 151(Suppl 3):U25–U37 - PubMed
-
- Sap J, Muñoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, Beug H, Vennström B1986. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature 324:635–640 - PubMed
-
- Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ, Evans RM1986. The c-erb-A gene encodes a thyroid hormone receptor. Nature 324:641–646 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

