Anticolon cancer activity of largazole, a marine-derived tunable histone deacetylase inhibitor
- PMID: 20739454
- PMCID: PMC2967399
- DOI: 10.1124/jpet.110.172387
Anticolon cancer activity of largazole, a marine-derived tunable histone deacetylase inhibitor
Abstract
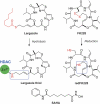
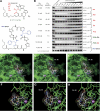
Histone deacetylases (HDACs) are validated targets for anticancer therapy as attested by the approval of suberoylanilide hydroxamic acid (SAHA) and romidepsin (FK228) for treating cutaneous T cell lymphoma. We recently described the bioassay-guided isolation, structure determination, synthesis, and target identification of largazole, a marine-derived antiproliferative natural product that is a prodrug that releases a potent HDAC inhibitor, largazole thiol. Here, we characterize the anticancer activity of largazole by using in vitro and in vivo cancer models. Screening against the National Cancer Institute's 60 cell lines revealed that largazole is particularly active against several colon cancer cell types. Consequently, we tested largazole, along with several synthetic analogs, for HDAC inhibition in human HCT116 colon cancer cells. Enzyme inhibition strongly correlated with the growth inhibitory effects, and differential activity of largazole analogs was rationalized by molecular docking to an HDAC1 homology model. Comparative genomewide transcript profiling revealed a close overlap of genes that are regulated by largazole, FK228, and SAHA. Several of these genes can be related to largazole's ability to induce cell cycle arrest and apoptosis. Stability studies suggested reasonable bioavailability of the active species, largazole thiol. We established that largazole inhibits HDACs in tumor tissue in vivo by using a human HCT116 xenograft mouse model. Largazole strongly stimulated histone hyperacetylation in the tumor, showed efficacy in inhibiting tumor growth, and induced apoptosis in the tumor. This effect probably is mediated by the modulation of levels of cell cycle regulators, antagonism of the AKT pathway through insulin receptor substrate 1 down-regulation, and reduction of epidermal growth factor receptor levels.
Figures
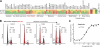




References
-
- Ackley DC, Rockich KT, Baker TR. ( 2004) Metabolic stability assessed by liver microsomes and hepatocytes, in Optimization in Drug Discovery: In Vitro Methods (Series: Methods in Pharmacology and Toxicology) (eds Yan Z, Caldwell JW.) pp 151– 162, Springer, New Jersey
-
- Baselga J. ( 2006) Targeting tyrosine kinases in cancer: the second wave. Science 312: 1175– 1178 - PubMed
-
- Baserga R. ( 2009) The insulin receptor substrate-1: a biomarker for cancer? Exp Cell Res 315: 727– 732 - PubMed
-
- Benjamini Y, Hochberg Y. ( 1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57: 289– 300
-
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. ( 2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19: 185– 193 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

