Spontaneous lymphatic vessel formation and regression in the murine cornea
- PMID: 20739466
- PMCID: PMC3053282
- DOI: 10.1167/iovs.10-5404
Spontaneous lymphatic vessel formation and regression in the murine cornea
Abstract
Purpose: Lymphatic dysfunctions are associated with many diseases, ranging from cancer metastasis to transplant rejection, for which there is little effective treatment. To date, there is no natural model with which to study lymphatic regression. This study was conducted to investigate whether murine cornea, an extensively exploited tissue for vascular studies, derives its lymphatic-free status from a natural regression mechanism. The differential behaviors between the lymphatic and blood vessels under normal development and inflammation conditions are also compared.
Methods: Normal mouse eyeballs or whole-mount corneas encompassing the entire course of corneal development and maturation and adult inflamed corneas were used for immunofluorescent microscopic studies.
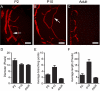
Results: The data demonstrated, for the first time, that mouse cornea was endowed with a significant number of lymphatic vessels that underwent spontaneous formation and regression during a critical period after birth, which was not observed for blood vessels. Because lymphatic growth can be reactivated in the adult cornea after inflammatory stimulation, the cornea thereby becomes the first tissue ever identified to have a full range of lymphatic plasticity.
Conclusions: These novel findings not only provide a new concept in defining the cornea and its related diseases, they also reveal a completely natural model with which to study both lymphatic regression and formation. It is hoped that further studies will divulge novel and potent pro- or anti-lymphatic factors to treat lymphatic disorders inside and outside the eye.
Figures





Similar articles
-
Differential distribution of blood and lymphatic vessels in the murine cornea.Invest Ophthalmol Vis Sci. 2010 May;51(5):2436-40. doi: 10.1167/iovs.09-4505. Epub 2009 Dec 17. Invest Ophthalmol Vis Sci. 2010. PMID: 20019372 Free PMC article.
-
Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation.Cornea. 2006 May;25(4):443-7. doi: 10.1097/01.ico.0000183485.85636.ff. Cornea. 2006. PMID: 16670483
-
Absence of blood and lymphatic vessels in the developing human cornea.Cornea. 2006 Jul;25(6):722-6. doi: 10.1097/01.ico.0000214230.21238.3d. Cornea. 2006. PMID: 17077668
-
Corneal lymphangiogenesis: implications in immunity.Semin Ophthalmol. 2009 May-Jun;24(3):135-8. doi: 10.1080/08820530902801320. Semin Ophthalmol. 2009. PMID: 19437348 Review.
-
Transgenic mouse models of angiogenesis and lymphangiogenesis.Int Rev Cell Mol Biol. 2008;266:1-35. doi: 10.1016/S1937-6448(07)66001-8. Int Rev Cell Mol Biol. 2008. PMID: 18544491 Review.
Cited by
-
Blockade of MMP-2 and MMP-9 inhibits corneal lymphangiogenesis.Graefes Arch Clin Exp Ophthalmol. 2017 Aug;255(8):1573-1579. doi: 10.1007/s00417-017-3651-8. Epub 2017 Jul 1. Graefes Arch Clin Exp Ophthalmol. 2017. PMID: 28669039
-
Specific role of lymphatic marker podoplanin in retinal pigment epithelial cells.Lymphology. 2010 Sep;43(3):128-34. Lymphology. 2010. PMID: 21226415 Free PMC article.
-
Increased lymphangiogenesis and hemangiogenesis in infant cornea.Lymphat Res Biol. 2011;9(2):109-14. doi: 10.1089/lrb.2011.0005. Lymphat Res Biol. 2011. PMID: 21688980 Free PMC article.
-
Matrix metalloproteinase 14 participates in corneal lymphangiogenesis through the VEGF-C/VEGFR-3 signaling pathway.Exp Ther Med. 2016 Oct;12(4):2120-2128. doi: 10.3892/etm.2016.3601. Epub 2016 Aug 22. Exp Ther Med. 2016. Retraction in: Exp Ther Med. 2025 Jan 27;29(3):59. doi: 10.3892/etm.2025.12809. PMID: 27698700 Free PMC article. Retracted.
-
Organogenesis and distribution of the ocular lymphatic vessels in the anterior eye.JCI Insight. 2020 Jul 9;5(13):e135121. doi: 10.1172/jci.insight.135121. JCI Insight. 2020. PMID: 32641580 Free PMC article.
References
-
- Folkman J, Kaipainen A. Genes tell lymphatics to sprout or not. Nat Immunol. 2004;5:11–12 - PubMed
-
- Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953 - PubMed
-
- Brown P. Lymphatic system: unlocking the drains. Nature. 2005;436:456–458 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

