Immune responses and therapeutic antitumor effects of an experimental DNA vaccine encoding human papillomavirus type 16 oncoproteins genetically fused to herpesvirus glycoprotein D
- PMID: 20739505
- PMCID: PMC2952997
- DOI: 10.1128/CVI.00264-10
Immune responses and therapeutic antitumor effects of an experimental DNA vaccine encoding human papillomavirus type 16 oncoproteins genetically fused to herpesvirus glycoprotein D
Abstract
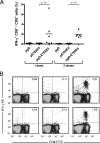
Recombinant adenovirus or DNA vaccines encoding herpes simplex virus type 1 (HSV-1) glycoprotein D (gD) genetically fused to human papillomavirus type 16 (HPV-16) oncoproteins (E5, E6, and E7) induce antigen-specific CD8(+) T-cell responses and confer preventive resistance to transplantable murine tumor cells (TC-1 cells). In the present report, we characterized some previously uncovered aspects concerning the induction of CD8(+) T-cell responses and the therapeutic anticancer effects achieved in C57BL/6 mice immunized with pgD-E7E6E5 previously challenged with TC-1 cells. Concerning the characterization of the immune responses elicited in mice vaccinated with pgD-E7E6E5, we determined the effect of the CD4(+) T-cell requirement, longevity, and dose-dependent activation on the E7-specific CD8(+) T-cell responses. In addition, we determined the priming/boosting properties of pgD-E7E6E5 when used in combination with a recombinant serotype 68 adenovirus (AdC68) vector encoding the same chimeric antigen. Mice challenged with TC-1 cells and then immunized with three doses of pgD-E7E6E5 elicited CD8(+) T-cell responses, measured by intracellular gamma interferon (IFN-γ) and CD107a accumulation, to the three HPV-16 oncoproteins and displayed in vivo antigen-specific cytolytic activity, as demonstrated with carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled target cells pulsed with oligopeptides corresponding to the H-2D(b)-restricted immunodominant epitopes of the E7, E6, or E5 oncoprotein. Up to 70% of the mice challenged with 5 × 10(5) TC-1 cells and immunized with pgD-E7E6E5 controlled tumor development even after 3 days of tumor cell challenge. In addition, coadministration of pgD-E7E6E5 with DNA vectors encoding pGM-CSF or interleukin-12 (IL-12) enhanced the therapeutic antitumor effects for all mice challenged with TC-1 cells. In conclusion, the present results expand our previous knowledge on the immune modulation properties of the pgD-E7E6E5 vector and demonstrate, for the first time, the strong antitumor effects of the DNA vaccine, raising promising perspectives regarding the development of immunotherapeutic reagents for the control of HPV-16-associated tumors.
Figures





References
-
- Aktas, E. U., C. Kucuksezer, S. Bilgic, G. Erten, and G. Deniz. 2009. Relationship between CD107a expression and cytotoxic activity. Cell. Immunol. 254:149-154. - PubMed
-
- Bosch, F. X., M. M. Manos, N. Muñoz, M. Sherman, A. M. Jansen, J. Peto, M. H. Schiffman, V. Moreno, R. Kurman, and K. V. Shah. 1995. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J. Natl. Cancer Inst. 87:796-802. - PubMed
-
- Braga, C. J. M., L. M. Massis, M. E. Sbrogio-Almeida, B. C. G. Alencar, B. Y. Bargieri, S. B. Boscardin, M. M. Rodrigues, and L. C. S. Ferreira. 2010. CD8+ T cell adjuvant effects of Salmonella FliCd flagellin in live vaccine vectors or as purified protein. Vaccine 28:1373-1382. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

