Glucose-induced ERM protein activation and translocation regulates insulin secretion
- PMID: 20739507
- PMCID: PMC2980361
- DOI: 10.1152/ajpendo.00199.2010
Glucose-induced ERM protein activation and translocation regulates insulin secretion
Abstract
A key step in regulating insulin secretion is insulin granule trafficking to the plasma membrane. Using live-cell time-lapse confocal microscopy, we observed a dynamic association of insulin granules with filamentous actin and PIP2-enriched structures. We found that the scaffolding protein family ERM, comprising ezrin, radixin, and moesin, are expressed in β-cells and target both F-actin and PIP2. Furthermore, ERM proteins are activated via phosphorylation in a glucose- and calcium-dependent manner. This activation leads to a translocation of the ERM proteins to sites on the cell periphery enriched in insulin granules, the exocyst complex docking protein Exo70, and lipid rafts. ERM scaffolding proteins also participate in insulin granule trafficking and docking to the plasma membrane. Overexpression of a truncated dominant-negative ezrin construct that lacks the ERM F-actin binding domain leads to a reduction in insulin granules near the plasma membrane and impaired secretion. Conversely, overexpression of a constitutively active ezrin results in more granules near the cell periphery and an enhancement of insulin secretion. Diabetic mouse islets contain less active ERM, suggestive of a novel mechanism whereby impairment of insulin granule trafficking to the membrane through a complex containing F-actin, PIP2, Exo70, and ERM proteins contributes to defective insulin secretion.
Figures

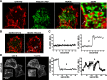




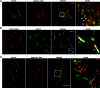


References
-
- Amieva MR, Litman P, Huang L, Ichimaru E, Furthmayr H. Disruption of dynamic cell surface architecture of NIH3T3 fibroblasts by the N-terminal domains of moesin and ezrin: in vivo imaging with GFP fusion proteins. J Cell Sci 112: 111–125, 1999. - PubMed
-
- Bittner MA, Holz RW. Phosphatidylinositol-4,5-bisphosphate: actin dynamics and the regulation of ATP-dependent and -independent secretion. Mol Pharmacol 67: 1089–1098, 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

