Pathogenesis and immune response of Crimean-Congo hemorrhagic fever virus in a STAT-1 knockout mouse model
- PMID: 20739514
- PMCID: PMC2953203
- DOI: 10.1128/JVI.01383-10
Pathogenesis and immune response of Crimean-Congo hemorrhagic fever virus in a STAT-1 knockout mouse model
Abstract
Tick-borne Crimean-Congo hemorrhagic fever virus (CCHFV) causes a severe hemorrhagic syndrome in humans but not in its vertebrate animal hosts. The pathogenesis of the disease is largely not understood due to the lack of an animal model. Laboratory animals typically show no overt signs of disease. Here, we describe a new small-animal model to study CCHFV pathogenesis that manifests clinical disease, similar to that seen in humans, without adaptation of the virus to the host. Our studies revealed that mice deficient in the STAT-1 signaling molecule were highly susceptible to infection, succumbing within 3 to 5 days. After CCHFV challenge, mice exhibited fever, leukopenia, thrombocytopenia, and highly elevated liver enzymes. Rapid viremic dissemination and extensive replication in visceral organs, mainly in liver and spleen, were associated with prominent histopathologic changes in these organs. Dramatically elevated proinflammatory cytokine levels were detected in the blood of the animals, suggestive of a cytokine storm. Immunologic analysis revealed delayed immune cell activation and intensive lymphocyte depletion. Furthermore, this study also demonstrated that ribavirin, a suggested treatment in human cases, protects mice from lethal CCHFV challenge. In conclusion, our data demonstrate that the interferon response is crucial in controlling CCHFV replication in this model, and this is the first study that offers an in-depth in vivo analysis of CCHFV pathophysiology. This new mouse model exhibits key features of fatal human CCHF, proves useful for the testing of therapeutic strategies, and can be used to study virus attenuation.
Figures

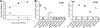


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

