Porcine reproductive and respiratory syndrome virus inhibits type I interferon signaling by blocking STAT1/STAT2 nuclear translocation
- PMID: 20739522
- PMCID: PMC2953160
- DOI: 10.1128/JVI.00655-10
Porcine reproductive and respiratory syndrome virus inhibits type I interferon signaling by blocking STAT1/STAT2 nuclear translocation
Erratum in
- J Virol. 2011 Jun;85(11):5705
Abstract
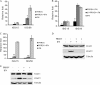
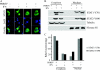
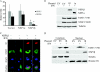
Type I interferons (IFNs) IFN-α/β play an important role in innate immunity against viral infections by inducing antiviral responses. Porcine reproductive and respiratory syndrome virus (PRRSV) inhibits the synthesis of type I IFNs. However, whether PRRSV can inhibit IFN signaling is less well understood. In the present study, we found that PRRSV interferes with the IFN signaling pathway. The transcript levels of IFN-stimulated genes ISG15 and ISG56 and protein level of signal transducer and activator of transcription 2 (STAT2) in PRRSV VR2385-infected MARC-145 cells were significantly lower than those in mock-infected cells after IFN-α treatment. IFN-induced phosphorylation of both STAT1 and STAT2 and their heterodimer formation in the PRRSV-infected cells were not affected. However, the majority of the STAT1/STAT2/IRF9 (IFN regulatory factor 9) heterotrimers remained in the cytoplasm of PRRSV-infected cells, which indicates that the nuclear translocation of the heterotrimers was blocked. Overexpression of NSP1β of PRRSV VR2385 inhibited expression of ISG15 and ISG56 and blocked nuclear translocation of STAT1, which suggests that NSP1β might be the viral protein responsible for the inhibition of IFN signaling. PRRSV infection in primary porcine pulmonary alveolar macrophages (PAMs) also inhibited IFN-α-stimulated expression of the ISGs and the STAT2 protein. In contrast, a licensed low-virulence vaccine strain, Ingelvac PRRS modified live virus (MLV), activated expression of IFN-inducible genes, including those of chemokines and antiviral proteins, in PAMs without the addition of external IFN and had no detectable effect on IFN signaling. These findings suggest that PRRSV interferes with the activation and signaling pathway of type I IFNs by blocking ISG factor 3 (ISGF3) nuclear translocation.
Figures
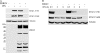


Similar articles
-
Porcine reproductive and respiratory syndrome virus Nsp1β inhibits interferon-activated JAK/STAT signal transduction by inducing karyopherin-α1 degradation.J Virol. 2013 May;87(9):5219-28. doi: 10.1128/JVI.02643-12. Epub 2013 Feb 28. J Virol. 2013. PMID: 23449802 Free PMC article.
-
Variable interference with interferon signal transduction by different strains of porcine reproductive and respiratory syndrome virus.Vet Microbiol. 2013 Oct 25;166(3-4):493-503. doi: 10.1016/j.vetmic.2013.07.022. Epub 2013 Jul 30. Vet Microbiol. 2013. PMID: 23953026
-
Porcine Reproductive and Respiratory Syndrome Virus nsp11 Antagonizes Type I Interferon Signaling by Targeting IRF9.J Virol. 2019 Jul 17;93(15):e00623-19. doi: 10.1128/JVI.00623-19. Print 2019 Aug 1. J Virol. 2019. PMID: 31092569 Free PMC article.
-
A Positive Feedback Amplifier Circuit That Regulates Interferon (IFN)-Stimulated Gene Expression and Controls Type I and Type II IFN Responses.Front Immunol. 2018 May 28;9:1135. doi: 10.3389/fimmu.2018.01135. eCollection 2018. Front Immunol. 2018. PMID: 29892288 Free PMC article. Review.
-
The unique role of STAT2 in constitutive and IFN-induced transcription and antiviral responses.Cytokine Growth Factor Rev. 2016 Jun;29:71-81. doi: 10.1016/j.cytogfr.2016.02.010. Epub 2016 Mar 18. Cytokine Growth Factor Rev. 2016. PMID: 27053489 Review.
Cited by
-
Ubiquitin-specific proteinase 1 stabilizes PRRSV nonstructural protein Nsp1β to promote viral replication by regulating K48 ubiquitination.J Virol. 2024 Mar 19;98(3):e0168623. doi: 10.1128/jvi.01686-23. Epub 2024 Feb 20. J Virol. 2024. PMID: 38376196 Free PMC article.
-
Porcine Reproductive and Respiratory Syndrome Virus Promotes SLA-DR-Mediated Antigen Presentation of Nonstructural Proteins To Evoke a Nonneutralizing Antibody Response In Vivo.J Virol. 2020 Oct 14;94(21):e01423-20. doi: 10.1128/JVI.01423-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32796065 Free PMC article.
-
Interferon Inducing Porcine Reproductive and Respiratory Syndrome Virus Vaccine Candidate Protected Piglets from HP-PRRSV Challenge and Evoke a Higher Level of Neutralizing Antibodies Response.Vaccines (Basel). 2020 Aug 31;8(3):490. doi: 10.3390/vaccines8030490. Vaccines (Basel). 2020. PMID: 32877992 Free PMC article.
-
Cellular poly(c) binding proteins 1 and 2 interact with porcine reproductive and respiratory syndrome virus nonstructural protein 1β and support viral replication.J Virol. 2011 Dec;85(24):12939-49. doi: 10.1128/JVI.05177-11. Epub 2011 Oct 5. J Virol. 2011. PMID: 21976648 Free PMC article.
-
Transactivation of programmed ribosomal frameshifting by a viral protein.Proc Natl Acad Sci U S A. 2014 May 27;111(21):E2172-81. doi: 10.1073/pnas.1321930111. Epub 2014 May 13. Proc Natl Acad Sci U S A. 2014. PMID: 24825891 Free PMC article.
References
-
- Albina, E., C. Carrat, and B. Charley. 1998. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. J. Interferon Cytokine Res. 18:485-490. - PubMed
-
- Banninger, G., and N. C. Reich. 2004. STAT2 nuclear trafficking. J. Biol. Chem. 279:39199-39206. - PubMed
-
- Bisbal, C., C. Martinand, M. Silhol, B. Lebleu, and T. Salehzada. 1995. Cloning and characterization of a RNAse L inhibitor. A new component of the interferon-regulated 2-5A pathway. J. Biol. Chem. 270:13308-13317. - PubMed
-
- Buddaert, W., K. Van Reeth, and M. Pensaert. 1998. In vivo and in vitro interferon (IFN) studies with the porcine reproductive and respiratory syndrome virus (PRRSV). Adv. Exp. Med. Biol. 440:461-467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

