Murine coronavirus receptors are differentially expressed in the central nervous system and play virus strain-dependent roles in neuronal spread
- PMID: 20739537
- PMCID: PMC2953140
- DOI: 10.1128/JVI.02688-09
Murine coronavirus receptors are differentially expressed in the central nervous system and play virus strain-dependent roles in neuronal spread
Abstract
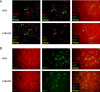
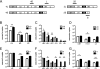
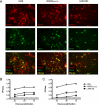
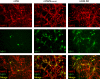
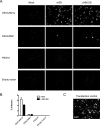
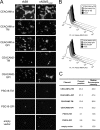
Coronavirus infection of the murine central nervous system (CNS) provides a model for studies of viral encephalitis and demyelinating disease. Mouse hepatitis virus (MHV) neurotropism varies by strain: MHV-A59 causes mild encephalomyelitis and demyelination, while the highly neurovirulent strain JHM.SD (MHV-4) causes fatal encephalitis with extensive neuronal spread of virus. In addition, while neurons are the predominant CNS cell type infected in vivo, the canonical receptor for MHV, the carcinoembryonic antigen family member CEACAM1a, has been demonstrated only on endothelial cells and microglia. In order to investigate whether CEACAM1a is also expressed in other cell types, ceacam1a mRNA expression was quantified in murine tissues and primary cells. As expected, among CNS cell types, microglia expressed the highest levels of ceacam1a, but lower levels were also detected in oligodendrocytes, astrocytes, and neurons. Given the low levels of neuronal expression of ceacam1a, primary neurons from wild-type and ceacam1a knockout mice were inoculated with MHV to determine the extent to which CEACAM1a-independent infection might contribute to CNS infection. While both A59 and JHM.SD infected small numbers of ceacam1a knockout neurons, only JHM.SD spread efficiently to adjacent cells in the absence of CEACAM1a. Quantification of mRNA for the ceacam1a-related genes ceacam2 and psg16 (bCEA), which encode proposed alternative MHV receptors, revealed low ceacam2 expression in microglia and oligodendrocytes and psg16 expression exclusively in neurons; however, only CEACAM2 mediated infection in human 293T cells. Therefore, neither CEACAM2 nor PSG16 is likely to be an MHV receptor on neurons, and the mechanism for CEACAM1a-independent neuronal spread of JHM.SD remains unknown.
Figures

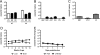
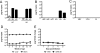
Similar articles
-
Pathogenesis of neurotropic murine coronavirus is multifactorial.Trends Pharmacol Sci. 2011 Jan;32(1):2-7. doi: 10.1016/j.tips.2010.11.001. Epub 2010 Dec 7. Trends Pharmacol Sci. 2011. PMID: 21144598 Free PMC article.
-
The spike glycoprotein of murine coronavirus MHV-JHM mediates receptor-independent infection and spread in the central nervous systems of Ceacam1a-/- Mice.J Virol. 2008 Jan;82(2):755-63. doi: 10.1128/JVI.01851-07. Epub 2007 Nov 14. J Virol. 2008. PMID: 18003729 Free PMC article.
-
Ceacam1a-/- mice are completely resistant to infection by murine coronavirus mouse hepatitis virus A59.J Virol. 2004 Sep;78(18):10156-65. doi: 10.1128/JVI.78.18.10156-10165.2004. J Virol. 2004. PMID: 15331748 Free PMC article.
-
Murine coronavirus neuropathogenesis: determinants of virulence.J Neurovirol. 2010 Nov;16(6):427-34. doi: 10.3109/13550284.2010.529238. Epub 2010 Nov 12. J Neurovirol. 2010. PMID: 21073281 Free PMC article. Review.
-
Pathogenesis of murine coronavirus in the central nervous system.J Neuroimmune Pharmacol. 2010 Sep;5(3):336-54. doi: 10.1007/s11481-010-9202-2. Epub 2010 Apr 6. J Neuroimmune Pharmacol. 2010. PMID: 20369302 Free PMC article. Review.
Cited by
-
Serum amyloid A: an ozone-induced circulating factor with potentially important functions in the lung-brain axis.FASEB J. 2017 Sep;31(9):3950-3965. doi: 10.1096/fj.201600857RRR. Epub 2017 May 22. FASEB J. 2017. PMID: 28533327 Free PMC article.
-
Pathogenesis of neurotropic murine coronavirus is multifactorial.Trends Pharmacol Sci. 2011 Jan;32(1):2-7. doi: 10.1016/j.tips.2010.11.001. Epub 2010 Dec 7. Trends Pharmacol Sci. 2011. PMID: 21144598 Free PMC article.
-
Viral inactivation of murine coronavirus via multiple gas plasma-derived reactive species.Redox Biol. 2025 May;82:103591. doi: 10.1016/j.redox.2025.103591. Epub 2025 Mar 10. Redox Biol. 2025. PMID: 40085974 Free PMC article.
-
Analysis of the host transcriptome from demyelinating spinal cord of murine coronavirus-infected mice.PLoS One. 2013 Sep 18;8(9):e75346. doi: 10.1371/journal.pone.0075346. eCollection 2013. PLoS One. 2013. PMID: 24058676 Free PMC article.
-
Cell-type-specific type I interferon antagonism influences organ tropism of murine coronavirus.J Virol. 2011 Oct;85(19):10058-68. doi: 10.1128/JVI.05075-11. Epub 2011 Jul 13. J Virol. 2011. PMID: 21752905 Free PMC article.
References
-
- Aloisi, F., F. Ria, G. Penna, and L. Adorini. 1998. Microglia are more efficient than astrocytes in antigen processing and in Th1 but not Th2 cell activation. J. Immunol. 160:4671-4680. - PubMed
-
- Banker, G., and K. Goslin (ed.). 1991. Culturing nerve cells. MIT Press, Cambridge, MA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

