Impaired orthotopic glioma growth and vascularization in transgenic mouse models of Alzheimer's disease
- PMID: 20739545
- PMCID: PMC2935547
- DOI: 10.1523/JNEUROSCI.2586-10.2010
Impaired orthotopic glioma growth and vascularization in transgenic mouse models of Alzheimer's disease
Abstract
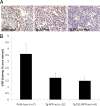
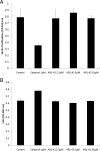
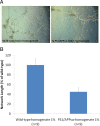
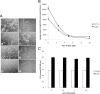
Alzheimer's disease (AD) is the most common form of dementia among the aging population and is characterized pathologically by the progressive intracerebral accumulation of beta-amyloid (Abeta) peptides and neurofibrillary tangles. The level of proangiogenic growth factors and inflammatory mediators with proangiogenic activity is known to be elevated in AD brains which has led to the supposition that the cerebrovasculature of AD patients is in a proangiogenic state. However, angiogenesis depends on the balance between proangiogenic and antiangiogenic factors and the brains of AD patients also show an accumulation of endostatin and Abeta peptides which have been shown to be antiangiogenic. To determine whether angiogenesis is compromised in the brains of two transgenic mouse models of AD overproducing Abeta peptides (Tg APPsw and Tg PS1/APPsw mice), we assessed the growth and vascularization of orthotopically implanted murine gliomas since they require a high degree of angiogenesis to sustain their growth. Our data reveal that intracranial tumor growth and angiogenesis is significantly reduced in Tg APPsw and Tg PS1/APPsw mice compared with their wild-type littermates. In addition, we show that Abeta inhibits the angiogenesis stimulated by glioma cells when cocultured with human brain microvascular cells on a Matrigel layer. Altogether our data suggest that the brain of transgenic mouse models of AD does not constitute a favorable environment to support neoangiogenesis and may explain why vascular insults synergistically precipitate the cognitive presentation of AD.
Figures

Similar articles
-
Reduction of beta-amyloid pathology by celastrol in a transgenic mouse model of Alzheimer's disease.J Neuroinflammation. 2010 Mar 8;7:17. doi: 10.1186/1742-2094-7-17. J Neuroinflammation. 2010. PMID: 20211007 Free PMC article.
-
Alzheimer's disease pathological lesions activate the spleen tyrosine kinase.Acta Neuropathol Commun. 2017 Sep 6;5(1):69. doi: 10.1186/s40478-017-0472-2. Acta Neuropathol Commun. 2017. PMID: 28877763 Free PMC article.
-
Impaired angiogenesis in a transgenic mouse model of cerebral amyloidosis.Neurosci Lett. 2004 Aug 5;366(1):80-5. doi: 10.1016/j.neulet.2004.05.017. Neurosci Lett. 2004. PMID: 15265595
-
APP transgenic modeling of Alzheimer's disease: mechanisms of neurodegeneration and aberrant neurogenesis.Brain Struct Funct. 2010 Mar;214(2-3):111-26. doi: 10.1007/s00429-009-0232-6. Epub 2009 Nov 29. Brain Struct Funct. 2010. PMID: 20091183 Free PMC article. Review.
-
Tg-SwDI transgenic mice: A suitable model for Alzheimer's disease and cerebral amyloid angiopathy basic research and preclinical studies.Exp Neurol. 2025 May;387:115189. doi: 10.1016/j.expneurol.2025.115189. Epub 2025 Feb 18. Exp Neurol. 2025. PMID: 39978567 Review.
Cited by
-
Brain microvasculature and hypoxia-related proteins in Alzheimer's disease.Int J Clin Exp Pathol. 2011 Aug 15;4(6):616-27. Epub 2011 Jun 18. Int J Clin Exp Pathol. 2011. PMID: 21904637 Free PMC article.
-
Human apolipoprotein E ɛ4 expression impairs cerebral vascularization and blood-brain barrier function in mice.J Cereb Blood Flow Metab. 2015 Jan;35(1):86-94. doi: 10.1038/jcbfm.2014.172. Epub 2014 Oct 22. J Cereb Blood Flow Metab. 2015. PMID: 25335802 Free PMC article.
-
The Physiological Roles of Amyloid-β Peptide Hint at New Ways to Treat Alzheimer's Disease.Front Aging Neurosci. 2018 Apr 25;10:118. doi: 10.3389/fnagi.2018.00118. eCollection 2018. Front Aging Neurosci. 2018. PMID: 29922148 Free PMC article. Review.
-
Oligomer-Aβ42 suppress glioma progression via potentiating phagocytosis of microglia.CNS Neurosci Ther. 2024 Jan;30(1):e14495. doi: 10.1111/cns.14495. Epub 2023 Oct 17. CNS Neurosci Ther. 2024. PMID: 37849438 Free PMC article.
-
Presenilin1 exerts antiproliferative effects by repressing the Wnt/β-catenin pathway in glioblastoma.Cell Commun Signal. 2020 Feb 11;18(1):22. doi: 10.1186/s12964-019-0501-9. Cell Commun Signal. 2020. PMID: 32046730 Free PMC article.
References
-
- Boscolo E, Folin M, Nico B, Grandi C, Mangieri D, Longo V, Scienza R, Zampieri P, Conconi MT, Parnigotto PP, Ribatti D. Beta amyloid angiogenic activity in vitro and in vivo. Int J Mol Med. 2007;19:581–587. - PubMed
-
- Bouras C, Kövari E, Herrmann FR, Rivara CB, Bailey TL, von Gunten A, Hof PR, Giannakopoulos P. Stereologic analysis of microvascular morphology in the elderly: Alzheimer disease pathology and cognitive status. J Neuropathol Exp Neurol. 2006;65:235–244. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
