In vivo NMDA receptor activation accelerates motor unit maturation, protects spinal motor neurons, and enhances SMN2 gene expression in severe spinal muscular atrophy mice
- PMID: 20739549
- PMCID: PMC6633349
- DOI: 10.1523/JNEUROSCI.1764-10.2010
In vivo NMDA receptor activation accelerates motor unit maturation, protects spinal motor neurons, and enhances SMN2 gene expression in severe spinal muscular atrophy mice
Abstract
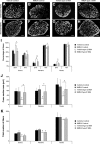
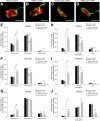
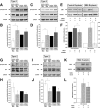
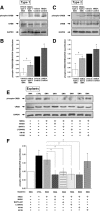
Spinal muscular atrophy (SMA), a lethal neurodegenerative disease that occurs in childhood, is caused by the misexpression of the survival of motor neuron (SMN) protein in motor neurons. It is still unclear whether activating motor units in SMA corrects the delay in the postnatal maturation of the motor unit resulting in an enhanced neuroprotection. In the present work, we demonstrate that an adequate NMDA receptor activation in a type 2 SMA mouse model significantly accelerated motor unit postnatal maturation, counteracted apoptosis in the spinal cord, and induced a marked increase of SMN expression resulting from a modification of SMN2 gene transcription pattern. These beneficial effects were dependent on the level of NMDA receptor activation since a treatment with high doses of NMDA led to an acceleration of the motor unit maturation but favored the apoptotic process and decreased SMN expression. In addition, these results suggest that the NMDA-induced acceleration of motor unit postnatal maturation occurred independently of SMN. The NMDA receptor activating treatment strongly extended the life span in two different mouse models of severe SMA. The analysis of the intracellular signaling cascade that lay downstream the activated NMDA receptor revealed an unexpected reactivation of the CaMKII/AKT/CREB (cAMP response element-binding protein) pathway that induced an enhanced SMN expression. Therefore, pharmacological activation of spinal NMDA receptors could constitute a useful strategy for both increasing SMN expression and limiting motor neuron death in SMA spinal cord.
Figures




References
-
- Allemand E, Batsché E, Muchardt C. Splicing, transcription, and chromatin: a ménage à trois. Curr Opin Genet Dev. 2008;18:145–151. - PubMed
-
- Andreassi C, Patrizi AL, Monani UR, Burghes AH, Brahe C, Eboli ML. Expression of the survival of motor-neuron (SMN) gene in primary neurons and increase in SMN levels by activation of the N-methyl-d-aspartate glutamate receptor. Neurogenetics. 2002;4:29–36. - PubMed
-
- Biondi O, Grondard C, Lécolle S, Deforges S, Pariset C, Lopes P, Cifuentes-Diaz C, Li H, della Gaspera B, Chanoine C, Charbonnier F. Exercise-induced activation of NMDA receptor promotes motor unit development and survival in a type 2 spinal muscular atrophy model mouse. J Neurosci. 2008;28:953–962. - PMC - PubMed
-
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
