The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses
- PMID: 20739552
- PMCID: PMC6633347
- DOI: 10.1523/JNEUROSCI.1491-10.2010
The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses
Abstract
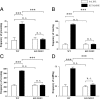
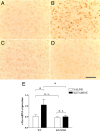
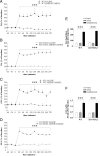
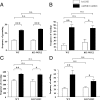
Subanesthetic doses of NMDA receptor antagonist ketamine induce schizophrenia-like symptoms in humans and behavioral changes in rodents. Subchronic administration of ketamine leads to loss of parvalbumin-positive interneurons through reactive oxygen species (ROS), generated by the NADPH oxidase NOX2. However, ketamine induces very rapid alterations, in both mice and humans. Thus, we have investigated the role of NOX2 in acute responses to subanesthetic doses of ketamine. In wild-type mice, ketamine caused rapid (30 min) behavioral alterations, release of neurotransmitters, and brain oxidative stress, whereas NOX2-deficient mice did not display such alterations. Decreased expression of the subunit 2A of the NMDA receptor after repetitive ketamine exposure was also precluded by NOX2 deficiency. However, neurotransmitter release and behavioral changes in response to amphetamine were not altered in NOX2-deficient mice. Our results suggest that NOX2 is a major source of ROS production in the prefrontal cortex controlling glutamate release and associated behavioral alterations after acute ketamine exposure. Prolonged NOX2-dependent glutamate release may lead to neuroadaptative downregulation of NMDA receptor subunits.
Figures

References
-
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. - PubMed
-
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
