Transcranial electric stimulation entrains cortical neuronal populations in rats
- PMID: 20739569
- PMCID: PMC2937280
- DOI: 10.1523/JNEUROSCI.5252-09.2010
Transcranial electric stimulation entrains cortical neuronal populations in rats
Abstract
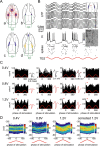
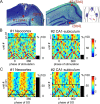
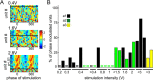
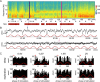
Low intensity electric fields have been suggested to affect the ongoing neuronal activity in vitro and in human studies. However, the physiological mechanism of how weak electrical fields affect and interact with intact brain activity is not well understood. We performed in vivo extracellular and intracellular recordings from the neocortex and hippocampus of anesthetized rats and extracellular recordings in behaving rats. Electric fields were generated by sinusoid patterns at slow frequency (0.8, 1.25 or 1.7 Hz) via electrodes placed on the surface of the skull or the dura. Transcranial electric stimulation (TES) reliably entrained neurons in widespread cortical areas, including the hippocampus. The percentage of TES phase-locked neurons increased with stimulus intensity and depended on the behavioral state of the animal. TES-induced voltage gradient, as low as 1 mV/mm at the recording sites, was sufficient to phase-bias neuronal spiking. Intracellular recordings showed that both spiking and subthreshold activity were under the combined influence of TES forced fields and network activity. We suggest that TES in chronic preparations may be used for experimental and therapeutic control of brain activity.
Figures


References
-
- Adey WR. Tissue interactions with nonionizing electromagnetic fields. Physiol Rev. 1981;61:435–514. - PubMed
-
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. - PubMed
-
- Barthó P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsáki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol. 2004;92:600–608. - PubMed
-
- Bawin SM, Sheppard AR, Mahoney MD, Adey WR. Influences of sinusoidal electric fields on excitability in the rat hippocampal slice. Brain Res. 1984;323:227–237. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
