Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice
- PMID: 20739601
- PMCID: PMC3350035
- DOI: 10.1152/jn.00563.2010
Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice
Abstract
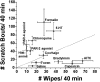
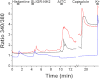
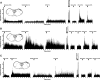
Intradermal cheek injection of pruitogens or algogens differentially elicits hindlimb scratching or forelimb wiping, suggesting that these behaviors distinguish between itch and pain. We studied whether pruritogens and algogens excite separate or overlapping populations of primary afferent and second-order trigeminal neurons in mice. Calcium imaging of primary sensory trigeminal ganglion (TG) cells showed that 15.4% responded to histamine, 5.8% to the protease-activated receptor (PAR)-2 agonist, 13.4% to allyl isothiocyanate (AITC), and 36.7% to capsaicin. AITC and/or capsaicin activated the vast majority of histamine- and PAR-2 agonist-sensitive TG cells. A chemical search strategy identified second-order neurons in trigeminal subnucleus caudalis (Vc) responsive to histamine, the PAR-2 agonist, or AITC. A minority of histamine or PAR-2 agonist-responsive Vc neurons responded to the other pruritogen, whereas a large majority of puritogen-responsive Vc neurons responded to capsaicin and/or AITC. A minority of AITC-responsive Vc neurons responded to pruritogens, whereas most responded to capsaicin. These data indicate that most primary and higher-order trigeminal sensory neurons are activated by both pruritic and algesic stimuli, although a minority exhibit selectivity. The results are discussed in terms of population codes for itch and pain that result in distinct behavioral responses of hindlimb scratching and forelimb wiping that are mediated at lumbar and cervical segmental levels, respectively.
Figures



References
-
- Akiyama T, Carstens MI, Carstens E. Differential itch- and pain-related behavioral responses and μ-opioid modulation in mice. Acta Derm Venereol In press 2010a - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

