PD-L1(hi) retinal pigment epithelium (RPE) cells elicited by inflammatory cytokines induce regulatory activity in uveitogenic T cells
- PMID: 20739617
- PMCID: PMC2996892
- DOI: 10.1189/jlb.0610332
PD-L1(hi) retinal pigment epithelium (RPE) cells elicited by inflammatory cytokines induce regulatory activity in uveitogenic T cells
Abstract
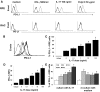
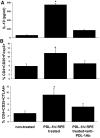
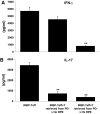
We previously reported that after exposure to inflammatory cytokines, such as IL-17 and IFN-γ, RPE cells express increased amounts of suppressor of cytokine signaling, leading to general suppression of the inflammatory response. Here, we demonstrate that RPE cells expressed increased levels of PD-L1 in response to IL-17, IFN-γ, or Poly I:C. These PD-L1(hi) RPE cells inhibited the pathogenic activities of IRBP-specific T cells, which usually induced uveitis when injected into naïve mice (EAU). The suppressed pathogenicity of these uveitogenic T cells after exposure to PD-L1(hi) RPE cells could be partially reversed by anti-PD-L1 antibodies. Nevertheless, IRBP-specific T cells pre-exposed to PD-L1(hi) RPE cells displayed substantial suppressor activity, which strongly inhibited the activation of fresh IRBP-Teffs in response to subsequent antigenic challenge and when transferred into naïve mice, inhibited the induction of EAU by IRBP-Teff transfer. These findings suggest that inflammatory cytokine-triggered up-regulation of PD-L1 on RPE constitutes a critical factor for inducing infiltrated uveitogenic T cells with regulatory activities, which may accelerate the natural resolution of T cell-mediated intraocular inflammation.
Figures



References
-
- Amadi-Obi A., Yu C. R., Liu X., Mahdi R. M., Clarke G. L., Nussenblatt R. B., Gery I., Lee Y. S., Egwuagu C. E. (2007) TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat. Med. 13, 711–718 - PubMed
-
- Ishida K., Panjwani N., Cao Z., Streilein J. W. (2003) Participation of pigment epithelium in ocular immune privilege. 3. Epithelia cultured from iris, ciliary body, and retina suppress T-cell activation by partially non-overlapping mechanisms. Ocul. Immunol. Inflamm. 11, 91–105 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

