Orai1 interacts with STIM1 and mediates capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells
- PMID: 20739625
- PMCID: PMC2980318
- DOI: 10.1152/ajpcell.00548.2009
Orai1 interacts with STIM1 and mediates capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells
Abstract
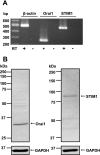
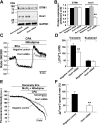
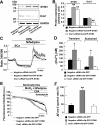
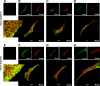
Previous studies in mouse pulmonary arterial smooth muscle cells (PASMCs) showed that cannonical transient receptor potential channel TRPC1 and stromal interaction molecule 1 (STIM1) mediate the sustained component of capacitative Ca(2+) entry (CCE), but the molecular candidate(s) that mediate the transient component of CCE remain unknown. The aim of the present study was to examine whether Orai1 mediates the transient component of CCE through activation of STIM1 in mouse PASMCs. In primary cultured mouse PASMCs loaded with fura-2, cyclopiazonic acid (CPA) caused a transient followed by a sustained rise in intracellular Ca(2+) concentration ([Ca(2+)](i)). The transient but not the sustained rise in [Ca(2+)](i) was partially inhibited by nifedipine. The nifedipine-insensitive transient rise in [Ca(2+)](i) and the increase in Mn(2+) quench of fura-2 fluorescence caused by CPA were both reduced in cells treated with Orai1 siRNA. These responses to CPA were further reduced in cells treated with Orai1 and STIM1 small interfering (si)RNA. Moreover, overexpression of STIM1 enhanced the rise in [Ca(2+)](i) and the increase in Mn(2+) quench of fura-2 fluorescence caused by CPA, and these responses were reduced in cells treated with Orai1 siRNA. RT-PCR revealed Orai1 and STIM1 mRNAs, and Western blot analysis identified Orai1 and STIM1 proteins in mouse PASMCs. Furthermore, Orai1 was found to coimmunoprecipitate with STIM1, and the precipitation level of Orai1 was increased in cells subjected to store-depletion. Immunostaining revealed colocalization of Orai1 and STIM1 proteins, and the colocalization of these proteins was more apparent after store-depletion. These data provide direct evidence that the transient component of CCE is mediated by Orai1 channel as a result of STIM1 activation in mouse PASMCs.
Figures


References
-
- Albert AP, Large WA. Store-operated Ca2+-permeable non-selective cation channels in smooth muscle cells. Cell Calcium 33: 345–356, 2003 - PubMed
-
- Baryshnikov SG, Pulina MV, Zulian A, Linde CI, Golovina VA. Orai1, a critical component of store-operated Ca2+ entry, is functionally associated with Na+/Ca2+ exchanger and plasma membrane Ca2+ pump in proliferating human arterial myocytes. Am J Physiol Cell Physiol 297: C1103–C1112, 2009 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

