Pilot pharmacologic randomized controlled trial for psychogenic nonepileptic seizures
- PMID: 20739647
- PMCID: PMC3013487
- DOI: 10.1212/WNL.0b013e3181f4d5a9
Pilot pharmacologic randomized controlled trial for psychogenic nonepileptic seizures
Abstract
Objective: There have been few treatment trials for psychogenic nonepileptic seizures (PNES). Some psychotherapies have been shown to improve PNES and comorbid symptom outcomes. We evaluated a pharmacologic intervention to test the hypothesis that sertraline would reduce PNES.
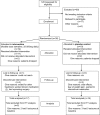
Methods: We conducted a pilot, double-blind, randomized, placebo-controlled trial in an academic medical hospital with epilepsy center outpatients. Subjects aged 18 to 65 years diagnosed with video-EEG-confirmed PNES were treated with flexible-dose sertraline or placebo over 12 weeks. Seizure calendars and symptom scales were charted prospectively. Secondary outcome measures included psychiatric symptom scales and psychosocial variables.
Results: Thirty-eight subjects enrolled, and 26 (68%) completed the trial. Thirty-three subjects with nonzero nonepileptic seizure rates at baseline were included in intent-to-treat analysis of the primary outcome. Subjects assigned to the sertraline arm experienced a 45% reduction in seizure rates from baseline to final visit (p = 0.03) vs an 8% increase in placebo (p = 0.78). Secondary outcome scales revealed no significant between-group differences in change scores from baseline to final visit, after adjustment for differences at baseline.
Conclusions: PNES were reduced in patients treated with a serotonin selective reuptake inhibitor, whereas those treated with placebo slightly increased. This study provides feasibility data for a larger-scale study.
Level of evidence: This study provides Class II evidence that flexible-dose sertraline up to a maximum dose of 200 mg is associated with a nonsignificant reduction in PNES rate compared with a placebo control arm (risk ratio 0.51, 95% confidence interval 0.25-1.05, p = 0.29), adjusting for differences at baseline.
Figures
References
-
- Gowers WR. Treatment: hysteroid attacks. In: Epilepsy and Other Chronic Convulsive Diseases: Their Causes, Symptoms, and Treatment. 2nd ed. London: Churchill; 1901:299–301.
-
- Shulman KI, Silver IL. Hysterical seizures as a manifestation of “depression” in old age. Can J Psychiatry 1985;30:278–280. - PubMed
-
- LaFrance WC Jr, Devinsky O. The treatment of nonepileptic seizures: historical perspectives and future directions. Epilepsia 2004;45(suppl 2):15–21. - PubMed
-
- LaFrance WC Jr, Barry JJ. Update on treatments of psychological nonepileptic seizures. Epilepsy Behav 2005;7:364–374. - PubMed
-
- Brooks JL, Goodfellow L, Bodde NM, Aldenkamp A, Baker GA. Nondrug treatments for psychogenic nonepileptic seizures: what's the evidence? Epilepsy Behav 2007;11:367–377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01AG016335/AG/NIA NIH HHS/United States
- V1R01-AR056834/AR/NIAMS NIH HHS/United States
- R01AA016799/AA/NIAAA NIH HHS/United States
- 1R01 AR056834-01S1/AR/NIAMS NIH HHS/United States
- 5R01CA123544/CA/NCI NIH HHS/United States
- R34MH079108/MH/NIMH NIH HHS/United States
- K23 NS045902/NS/NINDS NIH HHS/United States
- 5K23NS45902/NS/NINDS NIH HHS/United States
- R01 AA015950/AA/NIAAA NIH HHS/United States
- R01 MH071766/MH/NIMH NIH HHS/United States
- R01MH079153/MH/NIMH NIH HHS/United States
- U01CA150387-0/CA/NCI NIH HHS/United States
- R34 MH073625/MH/NIMH NIH HHS/United States
- R34 MH078855/MH/NIMH NIH HHS/United States
- P50CA84719/CA/NCI NIH HHS/United States
- R34MH08221/MH/NIMH NIH HHS/United States
- R21CA137211/CA/NCI NIH HHS/United States
- 5P20 RR024484/RR/NCRR NIH HHS/United States
- U01MH088278/MH/NIMH NIH HHS/United States
- R01DA023072/DA/NIDA NIH HHS/United States
- R01DA019558/DA/NIDA NIH HHS/United States
- R01NR010559/NR/NINR NIH HHS/United States
- 5U19AI070202/AI/NIAID NIH HHS/United States
- R01HL064342/HL/NHLBI NIH HHS/United States
- R01CA132854/CA/NCI NIH HHS/United States
- 1R01AA017895-01A2/AA/NIAAA NIH HHS/United States
- R34MH083065-01/MH/NIMH NIH HHS/United States
- R01DA023190/DA/NIDA NIH HHS/United States
- R34 MH070743-01/MH/NIMH NIH HHS/United States
- 5K23 NS045902/NS/NINDS NIH HHS/United States
- R01DA018079/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials