Cooperation between the septins and the actomyosin ring and role of a cell-integrity pathway during cell division in fission yeast
- PMID: 20739711
- PMCID: PMC2975288
- DOI: 10.1534/genetics.110.119842
Cooperation between the septins and the actomyosin ring and role of a cell-integrity pathway during cell division in fission yeast
Abstract
A major question about cytokinesis concerns the role of the septin proteins, which localize to the division site in all animal and fungal cells but are essential for cytokinesis only in some cell types. For example, in Schizosaccharomyces pombe, four septins localize to the division site, but deletion of the four genes produces only a modest delay in cell separation. To ask if the S. pombe septins function redundantly in cytokinesis, we conducted a synthetic-lethal screen in a septin-deficient strain and identified seven mutations. One mutation affects Cdc4, a myosin light chain that is an essential component of the cytokinetic actomyosin ring. Five others cause frequent cell lysis during cell separation and map to two loci. These mutations and their dosage suppressors define a signaling pathway (including Rho1 and a novel arrestin) for repairing cell-wall damage. The seventh mutation affects the poorly understood RNA-binding protein Scw1 and severely delays cell separation when combined either with a septin mutation or with a mutation affecting the septin-interacting, anillin-like protein Mid2, suggesting that Scw1 functions in a pathway parallel to that of the septins. Taken together, our results suggest that the S. pombe septins participate redundantly in one or more pathways that cooperate with the actomyosin ring during cytokinesis and that a septin defect causes septum defects that can be repaired effectively only when the cell-integrity pathway is intact.
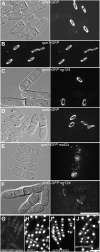
Figures





References
-
- Arellano, M., A. Durán and P. Pérez, 1997. Localisation of the Schizosaccharomyces pombe Rho1p GTPase and its involvement in the organisation of the actin cytoskeleton. J. Cell Sci. 110 2547–2555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

