Induced pluripotent stem cell-derived hepatocytes have the functional and proliferative capabilities needed for liver regeneration in mice
- PMID: 20739754
- PMCID: PMC2929736
- DOI: 10.1172/JCI43267
Induced pluripotent stem cell-derived hepatocytes have the functional and proliferative capabilities needed for liver regeneration in mice
Abstract
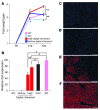
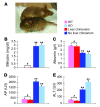
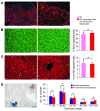
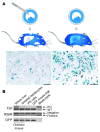
The ability to generate induced pluripotent stem (iPS) cells from a patient's somatic cells has provided a foundation for organ regeneration without the need for immune suppression. However, it has not been established that the differentiated progeny of iPS cells can effectively reverse failure of a vital organ. Here, we examined whether iPS cell-derived hepatocytes have both the functional and proliferative capabilities needed for liver regeneration in mice with fumarylacetoacetate hydrolase deficiency. To avoid biases resulting from random genomic integration, we used iPS cells generated without viruses. To exclude compensation by hepatocytes not derived from iPS cells, we generated chimeric mice in which all hepatocytes were iPS cell derived. In vivo analyses showed that iPS cells were intrinsically able to differentiate into fully mature hepatocytes that provided full liver function. The iPS cell-derived hepatocytes also replicated the unique proliferative capabilities of normal hepatocytes and were able to regenerate the liver after transplantation and two-thirds partial hepatectomy. Thus, our results establish the feasibility of using iPS cells generated in a clinically acceptable fashion for rapid and stable liver regeneration.
Figures
Comment in
-
From skin cells to hepatocytes: advances in application of iPS cell technology.J Clin Invest. 2010 Sep;120(9):3102-5. doi: 10.1172/JCI44422. Epub 2010 Aug 25. J Clin Invest. 2010. PMID: 20739747 Free PMC article. Review.
-
Modeling and therapy of human liver diseases using induced pluripotent stem cells: how far have we come?Hepatology. 2011 Feb;53(2):708-11. doi: 10.1002/hep.24143. Hepatology. 2011. PMID: 21274892 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

