Anaplasma phagocytophilum induces Ixodes scapularis ticks to express an antifreeze glycoprotein gene that enhances their survival in the cold
- PMID: 20739755
- PMCID: PMC2929727
- DOI: 10.1172/JCI42868
Anaplasma phagocytophilum induces Ixodes scapularis ticks to express an antifreeze glycoprotein gene that enhances their survival in the cold
Abstract
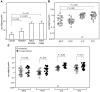
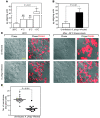
In the United States, Ixodes scapularis ticks overwinter in the Northeast and Upper Midwest and transmit the agent of human granulocytic anaplasmosis, Anaplasma phagocytophilum, among other pathogens. We now show that the presence of A. phagocytophilum in I. scapularis ticks increases their ability to survive in the cold. We identified an I. scapularis antifreeze glycoprotein, designated IAFGP, and demonstrated via RNAi knockdown studies the importance of IAFGP for the survival of I. scapularis ticks in a cold environment. Transfection studies also show that IAFGP increased the viability of yeast cells subjected to cold temperature. Remarkably, A. phagocytophilum induced the expression of iafgp, thereby increasing the cold tolerance and survival of I. scapularis. These data define a molecular basis for symbiosis between a human pathogenic bacterium and its arthropod vector and delineate what we believe to be a new pathway that may be targeted to alter the life cycle of this microbe and its invertebrate host.
Figures

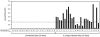



Comment in
-
Fitness and freezing: vector biology and human health.J Clin Invest. 2010 Sep;120(9):3087-90. doi: 10.1172/JCI44402. Epub 2010 Aug 25. J Clin Invest. 2010. PMID: 20739748 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

