The poly(A)-binding protein partner Paip2a controls translation during late spermiogenesis in mice
- PMID: 20739757
- PMCID: PMC2929737
- DOI: 10.1172/JCI43350
The poly(A)-binding protein partner Paip2a controls translation during late spermiogenesis in mice
Abstract
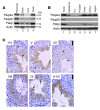
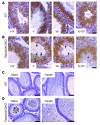
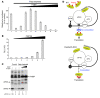
Translational control plays a key role in late spermiogenesis. A number of mRNAs encoding proteins required for late spermiogenesis are expressed in early spermatids but are stored as translationally inactive messenger ribonucleoprotein particles (mRNPs). The translation of these mRNAs is associated with shortening of their poly(A) tail in late spermiogenesis. Poly(A)-binding protein (Pabp) plays an important role in mRNA stabilization and translation. Three Pabp-interacting proteins, Paip1, Paip2a, and Paip2b, have been described. Paip2a is expressed in late spermatids. To investigate the role of Paip2 in spermiogenesis, we generated mice with knockout of either Paip2a or Paip2b and double-KO (DKO) mice lacking both Paip2a and Paip2b. Paip2a-KO and Paip2a/Paip2b-DKO mice exhibited male infertility. Translation of several mRNAs encoding proteins essential to male germ cell development was inhibited in late spermiogenesis in Paip2a/Paip2b-DKO mice, resulting in defective elongated spermatids. Inhibition of translation in Paip2a/Paip2b-DKO mice was caused by aberrant increased expression of Pabp, which impaired the interaction between eukaryotic initiation factor 4E (eIF4E) and the cap structure at the 5' end of the mRNA. We therefore propose a model whereby efficient mRNA translation in late spermiogenesis occurs at an optimal concentration of Pabp, a condition not fulfilled in Paip2a/Paip2b-DKO mice.
Figures




Comment in
-
Too much PABP, too little translation.J Clin Invest. 2010 Sep;120(9):3090-3. doi: 10.1172/JCI44091. Epub 2010 Aug 25. J Clin Invest. 2010. PMID: 20739750 Free PMC article.
Similar articles
-
Regulation of poly(A) binding protein function in translation: Characterization of the Paip2 homolog, Paip2B.RNA. 2006 Aug;12(8):1556-68. doi: 10.1261/rna.106506. Epub 2006 Jun 27. RNA. 2006. PMID: 16804161 Free PMC article.
-
PABP interacting protein 2A (PAIP2A) regulates specific key proteins during spermiogenesis in the mouse.Biol Reprod. 2012 Mar 30;86(3):95. doi: 10.1095/biolreprod.111.092619. Print 2012 Mar. Biol Reprod. 2012. PMID: 22190698
-
Too much PABP, too little translation.J Clin Invest. 2010 Sep;120(9):3090-3. doi: 10.1172/JCI44091. Epub 2010 Aug 25. J Clin Invest. 2010. PMID: 20739750 Free PMC article.
-
Regulation of poly(A)-binding protein through PABP-interacting proteins.Cold Spring Harb Symp Quant Biol. 2006;71:537-43. doi: 10.1101/sqb.2006.71.061. Cold Spring Harb Symp Quant Biol. 2006. PMID: 17381337 Review.
-
[Translational control by the poly(A) binding protein: a check for mRNA integrity].Mol Biol (Mosk). 2006 Jul-Aug;40(4):684-93. Mol Biol (Mosk). 2006. PMID: 16913227 Review. Russian.
Cited by
-
Roles of RNA-binding Proteins and Post-transcriptional Regulation in Driving Male Germ Cell Development in the Mouse.Adv Exp Med Biol. 2016;907:123-51. doi: 10.1007/978-3-319-29073-7_6. Adv Exp Med Biol. 2016. PMID: 27256385 Free PMC article.
-
Bioinformatic identification of novel elements potentially involved in messenger RNA fate control during spermatogenesis.Biol Reprod. 2012 Dec 13;87(6):138. doi: 10.1095/biolreprod.112.102434. Print 2012 Jun. Biol Reprod. 2012. PMID: 23053435 Free PMC article.
-
Linking spermatid ribonucleic acid (RNA) binding protein and retrogene diversity to reproductive success.Mol Cell Proteomics. 2013 Nov;12(11):3221-36. doi: 10.1074/mcp.M113.030585. Epub 2013 Aug 12. Mol Cell Proteomics. 2013. PMID: 23938467 Free PMC article.
-
Paip2 cooperates with Cbp80 at an active promoter and participates in RNA Polymerase II phosphorylation in Drosophila.FEBS Lett. 2019 May;593(10):1102-1112. doi: 10.1002/1873-3468.13391. Epub 2019 Apr 29. FEBS Lett. 2019. PMID: 31001806 Free PMC article.
-
Poly(A) binding proteins: are they all created equal?Wiley Interdiscip Rev RNA. 2013 Mar-Apr;4(2):167-79. doi: 10.1002/wrna.1151. Epub 2012 Dec 13. Wiley Interdiscip Rev RNA. 2013. PMID: 23424172 Free PMC article. Review.
References
-
- Kerr JB, Loveland KL, O’Bryan MK, de Kretser DM. Cytology of the testis and intrinsic control mechanisms. In: Neill JD, ed.Physiology of Reproduction . 3rd ed. Maryland Heights, Missouri, USA: Elsevier Press; 2006:1.
-
- Steger K. Transcriptional and translational regulation of gene expression in haploid spermatids. Anat Embryol (Berl). 1999;199(6):471–487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

