Hyposecretion of fluid from tracheal submucosal glands of CFTR-deficient pigs
- PMID: 20739758
- PMCID: PMC2929738
- DOI: 10.1172/JCI43466
Hyposecretion of fluid from tracheal submucosal glands of CFTR-deficient pigs
Abstract
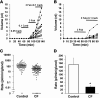
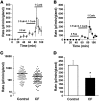
Cystic fibrosis (CF) results from mutations that disrupt CF transmembrane conductance regulator (CFTR), an anion channel found mainly in apical membranes of epithelial cells. CF leads to chronic infection of the airways with normally innocuous bacteria and fungi. Hypotheses to explain the pathophysiology of CF airways have been difficult to test because mouse models of CF do not develop human-like airway disease. The recent production of pigs lacking CFTR and pigs expressing the most common CF-causing CFTR mutant, DeltaF508, provide another model that might help clarify the pathophysiology of CF airway disease. Here, we studied individual submucosal glands from 1-day-old piglets in situ in explanted tracheas, using optical methods to monitor mucus secretion rates from multiple glands in parallel. Secretion rates from control piglets (WT and CFTR+/-) and piglets with CF-like disease (CFTR-/- and CFTR-/DeltaF508) were measured under 5 conditions: unstimulated (to determine basal secretion), stimulated with forskolin, stimulated with carbachol, stimulated with substance P, and, as a test for synergy, stimulated with forskolin and a low concentration of carbachol. Glands from piglets with CF-like disease responded qualitatively to all agonists like glands from human patients with CF, producing virtually no fluid in response to stimulation with forskolin and substantially less in response to all other agonists except carbachol. These data are a step toward determining whether gland secretory defects contribute to CF airway disease.
Figures



Comment in
-
Transgenic animals may help resolve a sticky situation in cystic fibrosis.J Clin Invest. 2010 Sep;120(9):3093-6. doi: 10.1172/JCI44235. Epub 2010 Aug 25. J Clin Invest. 2010. PMID: 20739746 Free PMC article.

